Full Text
Introduction
In 2016, globally there were an estimated 10.4 million incident cases of tuberculosis (ranging from 8.8 million to 12.2 million), equivalent to 140 cases per 100,000 population. Most of the estimated number of cases in 2016 occurred in the WHO South-East Asia Region (45%). The five countries that stood out, as having the largest number of incident cases were India, Indonesia, China, the Philippines and Pakistan, which together accounted for 56% of the global total. Of these, China, India and Indonesia alone accounted for 45% of global cases. Nigeria and South Africa each accounted for 4% of the global total [1].
India stands highest, in burden of tuberculosis (TB). The World Health Organisation (WHO) TB statistics for India for 2016, estimated 2.79 million cases for India. Incidence of multi-drug resistance tuberculosis (MDR TB) with rifampicin resistance is 11 per 100,000 population [2].
GeneXpert MTB/RIF assay (Cepheid, USA) is a rapid molecular test for diagnosis of TB recommended by WHO. It was initially recommended (in 2010) for diagnosis of pulmonary TB in adults. Since 2013, it has also been recommended for use in children and to diagnose specific forms of extrapulmonary TB (EPTB). The test has much better accuracy than sputum smear microscopy [1]. GeneXpert MB/RIF assay is currently used to detect Mycobacterium tuberculosis (MTB) complex DNA and its susceptibility to rifampicin (RMP) in a single reaction. It is based on seminested real-time PCR (rT-PCR) that targets the rpo-B gene hot spot region [3, 4]. Monoresistance to RMP is rare, however, >90% of RMP resistant isolates also exhibit resistance to isoniazid (INH). Therefore, the detection of RMP resistance may serve as surrogate marker of MDR Mycobacterium tuberculosis [5, 6]. The test is carried out within 2 hours in a disposable cartridge [7].
Early diagnosis is helpful for early patient management and successful patient outcomes. False-negative results and misdiagnosis of TB suspects are common in developing nations, as most TB control programmes use Ziehl-Neelsen (ZN) smear microscopy, which has poor sensitivity and multiple visits are required which leads to higher default. Mycobacterial culture, although considered as the gold standard but is slow and usually takes 2-6 weeks time to yield a final result and requires proper infrastructure and technical expertise [8-10]. Diagnosis of EPTB is difficult to establish due to the low number of bacteria in clinical specimens. Rapid and accurate diagnosis of pulmonary and EPTB is still a great challenge [11].
In the present study, aim is to study pulmonary and extrapulmonary specimens by AFB staining (Zeihl Neelsen method) and molecular method of detection by cartridge based nucleic acid amplification test (CB-NAAT) or GeneXpert MTB/RIF assay (Cepheid, USA) in patients attending to a tertiary care hospital.
AIM: 1. To detect MTB by GeneXpert MTB/RIF in inpatient (IP) and outpatient (OP) and to find out the incidence rate, 2. To find out predominant gender and age group for MTB in IP and OP, 3. To compare pulmonary and extrapulmonary AFB smear report with GeneXpert RIF/MTB assay, along with detection of rifampicin resistance.
Materials and method
The study was conducted in the Department of Microbiology at Krishna Institute of Medical Sciences Ltd., Secunderabad, Telangana. The study duration was one year (1 August 2016- 31 July 2017). It is a laboratory based prospective study. A total of 320 various clinical specimens of tuberculosis suspected patients from inpatients and outpatients were studied. A total of 198 pulmonary specimens (bronchial wash, pleural fluid, sputum, ET secretion and tracheal secretion) and a total of 122 extrapulmonary specimens (tissue biopsy, lymph nodes, ultrasound guided aspiration, FNAC, pus, CSF, drain fluid, ascitic fluid, urine) were included in the study.
Inclusion criteria: All pulmonary and extrapulmonary specimens from IP and OP were received with request of two tests: 1. AFB smear examination by Ziehl Neelsen method and 2. GeneXpert MTB/RIF assay, which were included in this study.
Exclusion criteria: Those specimens which had only one method, either GeneXpert or AFB smear by Ziehl Neelsen staining method were excluded from the study.
Ziehl Neelsen staining method for detection of MTB
The smear preparation was done in biosafety cabinet level 2B. For sputum specimens, a direct smear and a concentration method smear (by Petroff’s method) were prepared and heat fixed. Acid fast bacilli (AFB) staining was done by Ziehl Neelsen staining method and then visualised under 40x and 100x light microscope [12, 13]. Reporting of sputum is done as per RNTCP guidelines [13, 14].
GeneXpert MTB/RIF assay (Figure 1a,b)
It detects MTB DNA in under 2hrs and detects mutations that cause resistance to rifampicin (Figure 1). Sample reagent was added at a 2:1 ratio to clinical specimens. The closed specimen container was manually agitated twice during a 15-min period at room temperature, before 2 ml of the inactivated material was transferred to the test cartridge. The last result was used for the analysis [15].
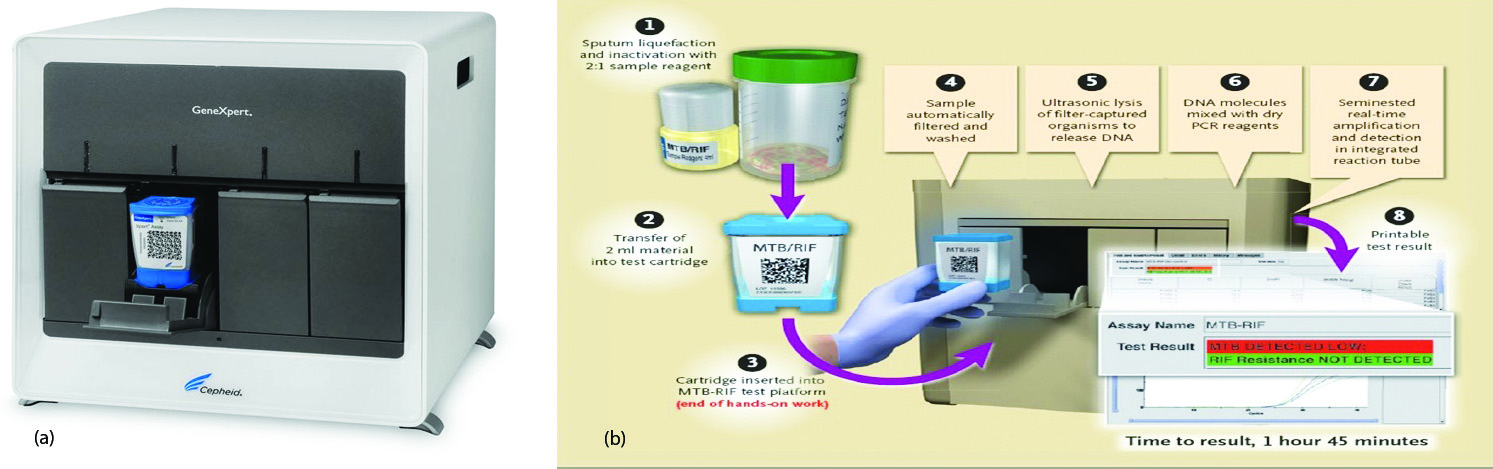
Figure 1: (a) GeneXpert MTB/RIF assay machine, (b) Stepwise procedure of GeneXpert.
A total of 320 specimens data were collected in excel format and analysed by using SPSS 20.
Results
Out of 320 clinical samples, 196 IP and 124 OP specimens were collected. Out of 196 IP, 25 MTB detected by GeneXpert and Out of 124 OP, 42 MTB were detected by GeneXpert MTB/RIF assay (Table 1).
Table 1: Mycobacterium tuberculosis detected by GeneXpert MTB/RIF assay in inpatient and outpatient specimens.
| |
|
IP; n=196 (61.25%)
|
OP; n=124 (38.75%)
|
|
M. tuberculosis not detected
|
171 (87.24%)
|
82 (66.13%)
|
|
M. tuberculosis detected
|
25 (12.76%)
|
42 (33.87%)
|
| |
Low
|
19
|
23
|
| |
Medium
|
2
|
12
|
| |
High
|
4
|
7
|
Among 196 IP patients, male was predominant gender (n=133) and among 124 OP patients, female was predominant gender (n=70) (Table 2).
Table 2: Mycobacterium tuberculosis detected by GeneXpert MTB/RIF assay in Males and Females.
| |
IP (n=196)
|
OP (n=124)
|
Total (n=320)
|
|
Male
|
133 (67.86%)
|
54 (43.55%)
|
187 (58.44%)
|
|
Female
|
63 (32.14%)
|
70 (56.45%)
|
133 (41.56%)
|
Among different age groups, 41-60years was most commonly affected age group followed by 21-40 years (Table 3).
Table 3: Mycobacterium tuberculosis detected by GeneXpert MTB/RIF assay age group wise.
|
Age group
|
IP (n= 196)
|
OP (n= 124)
|
Total (n=320)
|
|
Age
|
M
|
F
|
Age
|
M
|
F
|
|
<20
|
10
|
5
|
5
|
7
|
2
|
5
|
17 (5.31%)
|
|
21-40
|
46
|
30
|
16
|
54
|
11
|
43
|
100 (31.25%)
|
|
41-60
|
74
|
56
|
18
|
36
|
20
|
16
|
110 (34.38%)
|
|
61-80
|
59
|
38
|
21
|
22
|
15
|
7
|
81 (25.31%)
|
|
>81
|
7
|
4
|
3
|
5
|
4
|
1
|
12 (3.75%)
|
Abbreviations: M: Male; F: Female.
Among all IP patients, 8 were AFB smear positive and 188 were AFB smear negative. Among all OP patients, 11 were AFB smear positive and 113 were AFB smear negative. All smear positive and negative patients were subjected to GeneXpert MTB/ RIF assay (Table 4).
Table 4: Mycobacterium tuberculosis detected by AFB staining by Zeihl Neelsen method.
| |
AFB smear positive
|
AFB smear negative
|
|
IP
|
8 (4.08%)
|
188 (95.92%)
|
|
OP
|
11 (8.87%)
|
113 (91.13%)
|
|
Total
|
19 (5.94%)
|
301 (94.06%)
|
Among 8 smear positive IP patients, 7 patients were GeneXpert positive and one was negative. Among 188 smear negative IP patients, 18 patients were GeneXpert positive and 170 were negative. Among 11 smear positive OP patients, 11 patients were GeneXpert positive. Among 113 smear negative IP patients, 31 patients were GeneXpert positive and 82 were negative (Table 5).
Table 5: GeneXpert MTB/RIF assay result among AFB smear positive and AFB smear negative patients.
| |
IP patients
|
OP patients
|
|
AFB smear positive
|
AFB smear negative
|
AFB smear positive
|
AFB smear negative
|
|
GeneXpert not detected
|
1 (0.51%)
|
170 (86.74%)
|
0
|
82 (66.13%)
|
|
GeneXpert detected
|
7 (3.57%)
|
18 (9.18%)
|
11 (8.87%)
|
31 (25%)
|
| |
Low
|
2
|
17
|
4
|
19
|
| |
Medium
|
1
|
1
|
2
|
10
|
| |
High
|
4
|
0
|
5
|
2
|
Abbreviations: IP: inpatient; OP: outpatient; AFB: acid- vast bacilli.
Among various sample, 198 pulmonary specimens and 122 extrapulmonary specimens. Out of 198 pulmonary samples, 38 MTB were detected and among 122 extrapulmonary, 29 MTB were detected by GeneXpert MTB/RIF assay (Table 6).
Table 6: AFB smear report, GeneXpert report along with rifampicin resistance in pulmonary and extrapulmonary specimens.
|
S. No
|
Pulmonary
samples
(n=198;
61.88%)
|
Number
(%)
|
AFB
positive
report
|
GeneXpert positive report
|
Rifampicin
resistance
detected
|
|
No.
|
L
|
M
|
H
|
|
|
1
|
Bronchial
wash
|
100(50.50)
|
7
|
17
|
9
|
4
|
4
|
Not
detected
|
|
2
|
Pleural
fluid
|
62(31.31)
|
2
|
7
|
6
|
-
|
1
|
Not
detected
|
|
3
|
Sputum
|
31(15.66)
|
7
|
11
|
4
|
2
|
5
|
Not
detected
|
|
4
|
ET secretion
& Tracheal
secretion
|
5(2.53)
|
Nil
|
Nil
|
-
|
-
|
-
|
Not
detected
|
|
Total
|
198
|
16(8.08)
|
35
(17.68)
|
19
|
6
|
10
|
|
| |
|
S.No
|
Extra
pulmonary
samples
(n=122;
38.12%)
|
Number
(%)
|
AFB
positive
report
|
GeneXpert positive report
|
Rifampicin
resistance
detected
|
|
No.
|
L
|
M
|
H
|
|
|
1
|
Tissue biopsy
|
48(39.34%)
|
Nil
|
10
|
8
|
2
|
-
|
1
|
|
2
|
Lymph node
|
16(13.11)
|
1
|
5
|
4
|
1
|
-
|
Not
detected
|
|
3
|
Ultrasound
guided
aspiration
|
14(11.48)
|
Nil
|
5
|
3
|
2
|
-
|
Not
detected
|
|
4
|
FNAC
|
13(10.66)
|
Nil
|
4
|
3
|
1
|
-
|
Not
detected
|
|
5
|
Pus
|
12(9.84)
|
1
|
6
|
5
|
2
|
-
|
1
|
|
6
|
CSF
|
09(7.38)
|
Nil
|
Nil
|
-
|
-
|
-
|
Not
detected
|
|
7
|
Drain fluid
|
05(4.10)
|
1
|
1
|
1
|
-
|
-
|
Not
detected
|
|
8
|
Ascitic fluid
|
03(2.45)
|
Nil
|
Nil
|
-
|
-
|
-
|
Not
detected
|
|
9
|
Urine
|
02(1.64)
|
Nil
|
1
|
1
|
-
|
-
|
Not
detected
|
|
Total
|
122
|
3(2.45)
|
32
(26.23)
|
25
|
8
|
-
|
|
In this study, it was observed that patients can be categorized according to their AFB staining report and GeneXpert MTB/RIF assay report in both inpatients and outpatients (Table 7).
Table 7: Observation of AFB staining report & GeneXpert MTB/RIF assay report in both inpatients and outpatients.
| |
Pulmonary specimens
|
Extrapulmonary specimens
|
Total
|
|
IP
|
OP
|
IP
|
OP
|
|
AFB smear + GeneXpert +
|
6
|
9
|
2
|
2
|
19
|
|
AFB smear – GeneXpert +
|
18
|
10
|
5
|
21
|
54
|
|
AFB smear + GeneXpert -
|
1
|
0
|
0
|
0
|
1
|
|
AFB smear – GeneXpert -
|
115
|
39
|
49
|
43
|
246
|
|
Total
|
140
|
58
|
56
|
66
|
320
|
Abbreviations: IP: inpatient; OP: outpatient; AFB: acid- vast bacilli.
Discussion
In the prospective study, the diagnostic yield of GeneXpert to detect MTB in pulmonary and extrapulmonary specimens was evaluated and compared with AFB smear staining by Zeihl Neelsen staining. GeneXpert is a simple bench top point of care diagnostic assay that can be performed with minimal training. The results are available within 2 hours, much earlier than the culture which usually takes days to come positive [16-18].
Steingart et al. showed a systematic review of 27 studies, in which GeneXpert assay of respiratory specimens had a pooled sensitivity of 89% (95% CI: 85%–92%) and specificity of 99% (95% CI: 98%–99%) in the diagnosis of pulmonary TB [19]. Zeka et al. [20] showed that due to low number of bacilli in extrapulmonary samples, they are not detected by AFB staining frequently. They are detected by GeneXpert MTB/RIF assay, which were the limits of detection of the test, as comparable to the present study as shown in Table 6.
Out of 320 patients screened for AFB smear and GeneXpert MTB/RIF assay, inpatients were 196 (61.25%) and outpatients 124 (38.75%). Incidence rate for MTB detected by GeneXpert was higher in outpatients i.e. 42 (33.87%) followed by 25 inpatients (12.76%). Gender predominance seen in male (n=187; 58.44%) followed by female (n=133; 41.56%). The most common age group affected was 41-60 years (n=110; 34.38%) followed by 21-40 years (n=100; 31.25%), 61-80 years (n=81; 25.31%), <20years (n=17; 5.31%) and <80years (n=12; 3.75%). Out of 198 (61.88%) pulmonary specimens [bronchial wash(n=100; 50.50%), pleural fluid (n= 62; 31.31%), sputum (n=31; 15.66%), ET secretion and tracheal secretion (n=05; 2.53%) ] were detected by MTB/RIF assay followed by 122 (38.12%) extrapulmonary specimens [tissue biopsy (n=48; 39.34%), lymph nodes (n=16; 13.11%), ultrasound guided aspiration (n=14; 11.48%), FNAC (n=13; 10.66%), pus(n= 12; 9,84%), CSF (n=09; 7.38%), drain fluid (n=05; 4.10%), ascitic fluid (n=03; 2.45%), urine(n=02; 1.64%)].
Out of 198 pulmonary specimens, 16 (8.08%) were AFB smear positive and 35 (17.68%) were GeneXpert positive. One sample was found to be AFB staining positive and GeneXpert negative. The probable reason for false negative result would be PCR inhibitors present in the assay.
Out of 122 extrapulmonary specimens, 3 (2.45%) were AFB smear positive and 32 (26.23%) were GeneXpert positive. High level detection of MTB is observed in pulmonary specimens [sputum (5) followed by bronchial wash (4) and pleural fluid (1)]. In extrapulmonary specimens, low level detection of MTB is observed. High level detection was observed in AFB smears positive cases. Rifampicin resistance was seen in only two cases of extrapulmonary specimens (one in tissue biopsy and one in pus). However, GeneXpert does not eliminate the need of conventional microscopy, culture and anti-tubercular drug sensitivity that are required to monitor the progression of treatment and to detect resistance to drugs other than Rifampicin [21].
Conclusion
The GeneXpert MTB/RIF assay is a rapid and easy-to-perform fully automated Nucleic acid amplification test, which is extremely helpful in early diagnosis and to initiate the treatment of tuberculosis in extrapulmonary and pulmonary tuberculosis. It has a high capability to detect MTB complex DNA in AFB microscopy negative samples of pulmonary and extrapulmonary origin. The assay correctly detects the information from the rpoB hot spot region regarding rifampicin resistance. The early diagnosis by MTB/RIF assay and to initiate correct regimen with regular follow up would be helpful in management of tuberculosis patients.
Acknowledgements
Dr. Ashok R, Consultant and Dr. V. Lakshmi, Professor of Microbiology, Department of Laboratory services-Microbiology, Credence Diagnostic Centre, Hyderabad, and Department of Microbiology, Krishna Institute of Medical Sciences Ltd., Secunderabad, Telangana.
Conflict of interest
The authors declare no conflict of interest.
References
[1] Global tuberculosis report 2017. Geneva: World Health Organization; 2017. Available from: www.who.int/tb/publications/global_report/en/.
[2] TB India 2017 Revised National TB Control Programme Annual Status Report, New Delhi, 2017. Available from: www.tbcindia.nic.in/.
[3] Helb D, Jones M, Story E, Boehme C, Wallace E, et al. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J. Clin. Microbiol. 2010; 48(1):229–237.
[4] Van Rie A, Page-Shipp L, Scott L, Sanne I, Stevens W. Xpert® MTB/RIF for point-of care diagnosis of TB in high-HIV burden, resourcelimited countries: hype or hope? Expert Rev. Mol. Diagn. 2010; 10(7):937–946.
[5] Blakemore R, Story E, Helb D, Kop J, Banada P, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J. Clin Microbiol. 2010; 48(7):2495–2501.
[6] Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance, 2010; New England Journal of Medicine, 363: 1005–1015.
[7] Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J. Clin. Microbiol. 48(10):3551–3557.
[8] World Health Organization. Global tuberculosis report 2014. Geneva: WHO; 2014. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789 241564809_eng.pdf?ua=1.
[9] Evans CA. GenXpert – a game changer for tuberculosis control? PLOS MED. 2011; 8:e1001064.
[10] Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009; 58(01):7–10.
[11] Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013; 368(8): 745– 755.
[12] Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott’s Diagnostic Microbiology, 12th edition. The C.V Mosby Co. St.Louis. 2007, Chapter 45 Pg: 478–509.
[13]Tuberculosis India 2005. Technical and operational guidelines for tuberculosis control. New Delhi: Central Tuberculosis Division Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2005.
[14] Revised National Tuberculosis Control Programme (RNTCP). Manual for Laboratory Technicians. Central TB Division. Directorate General of Health Services, Ministry of Health and Family Welfare. New Delhi, India: 1999. Available from: http://www.tbcindia.org/LABMANUAL. pdf.
[15] Xpert MTB RIF kit insert. Available from: http://www.cepheid.com/manageddownloads/xpertmtb-rif-english-package-insert-301-1404-rev-b-february-2015.pdf.
[16] World Health Organization: Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert mtb/rif system. Policy statement 2011. Available from: http://whqlibdoc.who. int/publications/2011/9789241501545_eng.pdf.
[17] Van Rie A, Page-Shipp L, Scott L, Sanne I, Stevens W. Xpert(®) MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: hype or hope? Expert Rev Mol Diagn. 2010; 10(7):937–46.
[18] Agrawal M, Bajaj A, Bhatia V, Dutt S. Comparative Study of GeneXpert with ZN Stain and Culture in Samples of Suspected Pulmonary Tuberculosis. J Clin Diagn Res. 2016 May; 10(5): DC09–DC12.
[19] Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, et al. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;21(1): CD009593.
[20] Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011; 49(12):4138–41.
[21]International standard for tuberculosis care, 3rd edition, 2014. Available from: www.who.int/tb/ publications/standards-tb-care-2014/.