Orginal Research
2022
September
Volume : 10
Issue : 3
Cisplatin-induced ototoxicity in patients receiving concurrent chemoradiation in carcinoma cervix
Hanan M, Kutty CKK, Sameer P, Jayaraman MB
Pdf Page Numbers :- 141-146
Hanan M1, Kunhalan Kutty CK1, Sameer P2 and Jayaraman MB3,*
1Department of Radiotherapy, Government Medical College, Kozhikode, Kerala-673008, India
2Department of ENT, Government Medical College, Kozhikode, Kerala-673008, India
3Department of Radiation Oncology, Government Medical College, Thrissur, M. G. Kavu, Kerala-680596, India
*Corresponding author: Dr. Jayaraman MB, Associate professor, Department of Radiation Oncology, Government Medical College, Thrissur, M. G. Kavu, Kerala-680596, India. Email: drjayaramanmb@gmail.com
Received 21 March 2022; Revised 17 May 2022; Accepted 25 May 2022; Published 6 June 2022
Citation: Hanan M, Kutty CKK, Sameer P, Jayaraman MB. Cisplatin-induced ototoxicity in patients receiving concurrent chemoradiation in carcinoma cervix. J Med Sci Res. 2022; 10(3):141-146. DOI: http://dx.doi.org/10.17727/JMSR.2022/10-26
Copyright: © 2022 Hanan M et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Weekly cisplatin at 40mg/m2 is the most common regimen followed in our institution. However, patients receiving cisplatin can experience high-frequency hearing loss due to its expansive toxicity profile, a side effect known as ototoxicity. The dearth of information on the extent and severity of cisplatin-associated ototoxicity prevents implementing a context-specific audiological monitoring program. The study aimed to determine the extent and severity of cisplatin ototoxicity in patients receiving concurrent chemoradiation in carcinoma cervix.
Methods: Otoscopic examinations and audiological investigations were undertaken at regular intervals in patients undergoing concurrent chemoradiation for carcinoma cervix with single-agent cisplatin in the Department of Radiation Oncology, with the aid of the Department of ENT. All the audiological investigations were provided free of cost. Sixty-six patients who met the inclusion criteria were sent for audiological evaluation before the start of chemotherapy, immediately after chemotherapy completion and after one month following chemotherapy.
Results: The study found that cisplatin was associated with high frequency, predominantly bilateral sensorineural hearing loss. Among 66 patients, seven patients (11%) developed cisplatin-induced hearing loss. However, most of the patients developed mild and minimal hearing loss.
Conclusion: The study concluded that patients receiving concurrent chemoradiation for carcinoma cervix with cisplatin developed predominantly bilateral, high-frequency sensorineural hearing loss. However, most patients developed minimal or mild hearing loss.
Keywords: carcinoma cervix; cisplatin; ototoxicity; hearing loss
Full Text
Introduction
Carcinoma cervix is the most common gynaecological cancer and the 4th most common malignancy in women. Around 5,70,000 new cases were reported in the world. 90% of deaths occur in low and middle-income countries. In India, it is the second most common malignancy in women. Around 1,22,844 new cases were reported, in which 67,477 died due to cancer [1]. Incidence varies worldwide, with the highest rates in Latin America and the lowest prevalence among Jewish women in Israel. The human papillomavirus plays an important role in the genesis of cervix cancer and is observed in 90% of all women with cervix cancer [2]. The use of cervical screening has greatly reduced the incidence of invasive cervical cancer in western countries. Still, it continues to be a major cause of cancer mortality in developing countries like India because most patients have locally advanced disease at presentation [3]. The prognosis of cervical cancer is favorable, with an approximately 80–90% 5-year survival rate in early-stage disease. However, the advanced disease carries a poor prognosis [4]. Patients with early-stage disease (IB non-bulky and stage IIA) can expect a cure with either radical hysterectomy or radical radiotherapy. The standard treatment for stage IIB to IVA cervical cancer has been radical radiotherapy alone, followed by brachytherapy. In five randomized clinical trials, which consistently showed improved survival in patients treated with cisplatin-based chemoradiation, the U.S. National Cancer Institute (NCI) announced in 1999 that strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiotherapy in women who require radiotherapy for treatment of cervical cancer [5].
Cisplatin is a platinum analogue that binds to the N7 position of guanine and adenine. Dose-limiting side effects of cisplatin include nephrotoxicity, neurotoxicity and ototoxicity. While nephrotoxicity can, to some extent, be reversed by increasing saline hydration as well as mannitol diuresis, there are no known cures or preventative treatments available for ototoxicity and neurotoxicity. In the auditory system, the primary site of cisplatin toxicity is the outer hair cells, with the basal turn of the cochlea appearing to be most affected [6]. However, if cisplatin administration continues, damage to more apical areas is likely to occur [7]. Therefore, the initial manifestation of cisplatin-associated ototoxicity is the elevation of high-frequency audiometric thresholds [8]. Cisplatin-associated ototoxicity usually manifests as irreversible, progressive, bilateral, high-frequency sensorineural hearing loss associated with tinnitus [9]. The degree of hearing loss is often variable and is related to the dose. Ototoxicity poses a major problem to the patient receiving cisplatin chemotherapy, as the quality of life during and after receiving such therapy can be negatively affected due to hearing loss resulting in social, emotional and vocational difficulties. Therefore, given these negative attributes, as a result of cisplatin, one must ensure operational processes that minimize the resulting co-morbidities from using such drug regimens. An audiological monitoring program can avert, to a large extent, the reduced quality of life due to hearing loss since patients on such drugs can be identified early, counselled, monitored and managed appropriately through interventions in a logical, systematic way and coherent manner.
The study aimed to determine the extent and severity of cisplatin ototoxicity in patients receiving concurrent chemoradiation in carcinoma cervix.
Materials and methods
This prospective study was conducted in the Department of Radiation Oncology, Government Medical College, Kozhikode, from January 2019 to June 2020 in patients receiving concurrent chemoradiation in carcinoma cervix after ethical committee approval.
Inclusion criteria: Female patients in age group between 18 to 7 o years, creatining clearance more than 60ml/min and with ECOG performance status 0, 1 and 2.
Exclusion criteria: Patients with history of conductive/mixed pathology or any other sensorineural hearing loss, previous head and neck irradiation, brain metastasis, impaired renal function, patients with history of cisplatin chemotherapy, history of medical conditions such as tuberculosis and malaria (medications used in the treatment of these are ototoxic).
All patients meeting the inclusion criteria were included in the study. Patients were started on weekly cisplatin at 40mg/m2 after checking metabolic parameters with pelvic irradiation. Before beginning chemotherapy, the patients underwent otoscopic examination, PureTone audiometry, tympanometry and distortion product otoacoustic emissions (DPOAE) before starting chemotherapy, immediately after chemotherapy completion and one month after chemotherapy completion in both right ear and left ear. The frequency spectrum of hearing loss was assessed in each subject. Based on the results, patients were graded according to Goodman’s classification of hearing loss.
The data were analyzed with appropriate statistical tests using the SPSS version18. Frequencies and percentages of all variables were obtained. The chi-square test was used for qualitative variables. A p-value <0.05 was considered significant.
Results
All 66 patients included in the study had a normal otoscopic examination before starting chemotherapy, after completion, and after one month of chemotherapy in the right ear.
The right ear had normal tympanometry (A-type) before starting chemotherapy, after completion and after one month of chemotherapy completion.
The left ear had normal tympanometry (A-type) before starting chemotherapy, after completion and after one month of chemotherapy completion.
All 66 patients had a normal PureTone audiometry in the right ear before starting chemotherapy. Out of 66 patients who underwent PureTone audiometry in the right ear, 4 (6.1%) patients developed minimal hearing loss, and 2 (3%) patients developed mild hearing loss after completion of chemotherapy. Of 66 patients, 60 (90.9%) had a normal PureTone audiometry. The result was consistent after one month of chemotherapy.
All 66 patients had a normal PureTone audiometry in the left ear before starting chemotherapy. Out of 66 patients who underwent PureTone audiometry in the left ear, four patients (6.1%) developed minimal hearing loss and three patients (4.5%) developed mild hearing loss after completion of chemotherapy. Fifty-nine (89.4%) patients had a normal PureTone audiometry. The result was consistent after one month of chemotherapy (Table 1).
Table 1: Comparison of Puretone audiometry (PTA).
|
Ear
|
Group
|
Normal
|
Minimal hearing loss
|
Mild hearing loss
|
P value
|
|
Right
|
Pre-chemo
|
66
|
100%
|
0
|
0%
|
0
|
0%
|
0.172
|
|
Immediate post chemo
|
60
|
90.90%
|
4
|
6.10%
|
2
|
3.00%
|
|
Post chemo one month
|
60
|
90.90%
|
4
|
6.10%
|
2
|
3.00%
|
|
Left
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0
|
0%
|
0.110
|
|
Immediate post chemo
|
59
|
89.40%
|
4
|
6.10%
|
3
|
4.50%
|
|
Post chemo one month
|
59
|
89.40%
|
4
|
6.10%
|
3
|
4.50%
|
All 66 patients had a normal DPOAE in the right ear before chemotherapy. However, out of 66 patients, 2 (3%) had absent DPOAE after chemotherapy and one-month post-chemotherapy.
All 66 patients had a normal DPOAE in the left ear before chemotherapy. However, out of 66 patients, 3 (4.5%) had absent DPOAE after chemotherapy and one-month post-chemotherapy (Table 2).
Table 2: Comparison of DPOAE.
|
Ear
|
Group
|
Present
|
Absent
|
P value
|
|
Right
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0.360
|
|
Immediate post chemo
|
64
|
97.00%
|
2
|
3.00%
|
|
Post chemo one month
|
64
|
97.00%
|
2
|
3.00%
|
|
Left
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0.213
|
|
Immediate post chemo
|
63
|
95.50%
|
3
|
4.50%
|
|
Post chemo one month
|
63
|
95.50%
|
3
|
4.50%
|
All 66 patients had normal hearing before chemotherapy. However, six patients (9.1%) developed a sloping pattern of hearing loss after chemotherapy. All 66 patients included in the study had a normal otoscopic examination before starting chemotherapy, after completion, and after one month of chemotherapy in the left ear.
All 66 patients had normal hearing before chemotherapy. After chemotherapy, seven patients (10.6%) developed a sloping pattern of hearing loss. Before starting chemotherapy, all 66 patients had normal hearing sensitivity. Immediately after chemotherapy completion, six patients (9.1%) developed bilateral hearing loss. Only one patient (1.5%) developed unilateral hearing loss. Fifty-nine patients (89.4%) had a normal hearing sensitivity. The results were found to be similar after a one-month follow-up (Tables 3 and 4).
Table 3: Comparison of hearing loss.
|
Ear
|
Group
|
Present
|
Absent
|
P value
|
|
Right
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0.041
|
|
Immediate post chemo
|
60
|
90.90%
|
6
|
9.10%
|
|
Post chemo one month
|
60
|
90.90%
|
6
|
9.10%
|
|
Left
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0.023
|
|
Immediate post chemo
|
59
|
89.40%
|
7
|
10.60%
|
|
Post chemo one month
|
59
|
89.40%
|
7
|
10.60%
|
Table 4: Bilateral and unilateral hearing loss.
|
Group
|
Normal hearing
|
Bilateral Hearing Loss
|
Unilateral Hearing Loss
|
Total
|
P value
|
|
Pre chemo
|
66
|
100%
|
0
|
0%
|
0
|
0%
|
66
|
100%
|
0.11
|
|
Immediate post chemo
|
59
|
89.40%
|
4
|
6.10%
|
3
|
4.50%
|
66
|
100%
|
|
Post chemo one month
|
59
|
89.40%
|
4
|
6.10%
|
3
|
4.50%
|
66
|
100%
|
Before starting chemotherapy, all patients had normal hearing sensitivity. However, seven patients (10.6%) developed sensorineural hearing loss immediately after chemotherapy completion. This result was consistent after one month of follow-up (Figure 1).
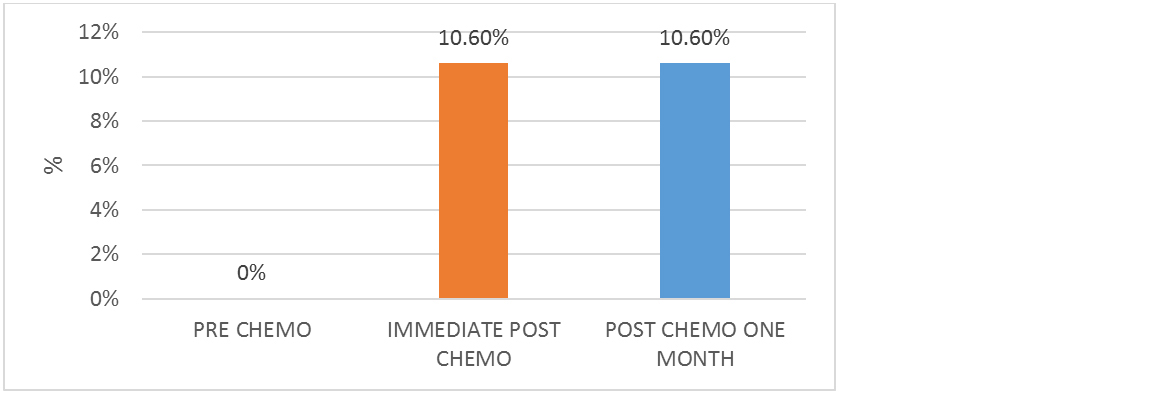
Figure 1: Cisplatin-induced hearing loss.
Discussion
The study assessed and analyzed the audiological functions of 66 patients undergoing concurrent chemoradiation with cisplatin for carcinoma cervix. All patients were above the age of 40. All received four cycles of weekly cisplatin at 40mg/m2. None of the patients had any treatment break. Patients with diabetes mellitus were excluded from the study as a confounding factor in the development of ototoxicity [10]. Patients who met the inclusion criteria were interviewed and evaluated for auditory functions before starting chemotherapy, immediately after completion of chemotherapy and after a month following chemotherapy completion. Audiological evaluations included an otoscopic examination, tympanometry, DPOAE and PureTone audiometry. In addition, PureTone audiometry frequencies of 250-8000Hz were tested. Hearing sensitivity was graded into normal hearing sensitivity or mild, moderate, moderately severe, severe and profound hearing loss based on Goodman’s classification of hearing loss [11]. DPOAE was tested based on if it was present or absent. All 66 patients tested before starting chemotherapy had normal audiological functions and hearing sensitivity. Immediate post-chemotherapy, all patients had normal otoscopic examination and tympanometry in both ears. This result was expected as cisplatin had no adverse effects on external or middle ear function. Further, they underwent DPOAE and PureTone audiometry to assess the degree and type of sensorineural hearing loss.
Two patients had mild hearing loss in the right ear, and four had minimal hearing loss. All the other 60 patients had a normal PureTone audiometry. This difference in the grade of hearing loss could probably be due to the genetic susceptibility of cisplatin ototoxicity [12]. Most frequencies above 4000Hz were affected. Two patients with mild hearing loss had absent DPOAE. In all the other patients tested, DPOAE was present. Six patients with abnormal PureTone audiometry had a sloping pattern of hearing loss [13]. All the other patients had normal hearing sensitivity.
Three patients in the left ear had mild hearing loss, and four had minimal hearing loss. All the other 59 patients tested had a normal PureTone audiometry. Most frequencies above 4000Hz were affected. Three patients with mild hearing loss had absent DPOAE. In all the other patients tested DPOAE was present. Seven patients with abnormal PureTone audiometry had a sloping pattern of hearing loss. All the other patients had normal hearing sensitivity.
Cisplatin-induced hearing loss often is permanent and bilaterally symmetric [14]. The results showed that 6 out of 66 patients (9.1%) developed bilateral hearing loss. Only one patient had an isolated left-sided hearing loss. There could be asymmetry in hearing patterns, as observed in studies [15]. The study observed that 7 out of 66 patients (11%) developed cisplatin-induced hearing loss, mostly involving frequencies above 4000Hz. This correlated to the findings by Dutta et al. [16], where 15% of patients who were subjected to chemotherapy based on cisplatin developed high-frequency sensorineural hearing loss. These 66 patients were followed up post one month after chemotherapy completion. The results were similar to those observed immediately after chemotherapy, providing a small insight into the irreversible nature of cisplatin ototoxicity.
Cisplatin-induced ototoxicity usually manifests as irreversible, progressive, bilateral high-frequency hearing loss associated with tinnitus [17]. However, a longer follow-up is required to prove the progressive nature of hearing loss.
This low incidence of cisplatin ototoxicity could be due to the lower dose (40mg/m2) of cisplatin. Studies have shown that increasing cumulative cisplatin dose was associated with a higher incidence of cisplatin ototoxicity [18]. Almost one in five (18%) patients had severe to profound hearing loss at doses more than 300mg/m2 [19]. Although the ototoxicity of cisplatin has been acknowledged, the survival benefit of cisplatin cannot be underwhelmed. The survival benefit of concurrent chemoradiation with cisplatin has been validated in multiple phases 3 trials. Based on the Gynec Oncology Group (GOG) trials GOG 123, GOG 85, GOG 120, GOG109, and RTOG 9001, the US National cancer institute (NCI) in February 1999 issued an alert that chemoradiation should be considered for all patients with cervical cancer over radiotherapy alone [20-24]. These studies showed an overall survival benefit of around 10-15%. A 2005 update of a meta-analysis of concomitant chemotherapy and radiation therapy found 24 trials and concluded that chemoradiation improves overall survival and PFS, with absolute benefits of 10% and 13%, respectively [25]. Similarly, a 2008 meta-analysis of the 13 trials that compared chemoradiotherapy to radiation found a 6% improvement in 5-year survival with concurrent chemoradiation (HR 0.81, P <0.001). The effect was attributed to a reduction in both local and distant recurrence [26]. A multi-institutional study from India also showed a significant survival advantage of chemoradiation over radiation alone, with a 5-year cumulative survival of 70.2% v 47.3% [27].
Hence, considering the survival benefit of concurrent chemoradiation in cervical cancer, the minimal and mild hearing loss induced by cisplatin is acceptable. However, these patients should undergo a periodic hearing evaluation to know the impact on quality of life. However, minimal and mild hearing loss may not require hearing aids if hearing loss progresses; hearing devices may be required.
Conclusion
The study concluded that in carcinoma cervix patients undergoing concurrent chemoradiation with cisplatin, 11% of patients developed high frequency, predominantly bilateral sensorineural hearing loss. However, most of the patients developed minimal and mild hearing loss. Therefore, it also emphasizes doing routine clinical and audiological evaluations before starting chemotherapy, between and after completion of chemotherapy in carcinoma cervix patients undergoing platinum-based concurrent chemoradiation in order to enhance the quality of life of patients.
Limitations
Conventional pure-tone audiometry (250 Hz to 8000Hz) was used as our institution did not have high-frequency audiometry (up to 16,000 Hz). The high-frequency PureTone audiometry would have better results as ototoxicity is more profound at high frequencies due to greater damage at the basal turn of the cochlea. The effect of cisplatin for different dosage schedules could not be assessed as all patients were given weekly cisplatin at 40mg/m2. An interim evaluation was not included in the study, which would have helped assess the cumulative dose of cisplatin that affected the cochlea. This data was collected in tertiary care centre in limited patients, multi-centre with more patients gives precise results.
Conflicts of interest
The authors declare no conflicts of interest.
References
[1] Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015; 7:405–414.
[2] Bergeron C. HPV typing in screening and diagnosis of precursor lesions of cervical carcinoma. Virologie. 2001; 5(6):431–438.
[3] Nour NM. Cervical cancer: a preventable death. Rev Obstet Gynecol. 2009; 2(4):240–244.
[4] Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999; 340(15):1154–1161.
[5] Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18(8):1606–1613.
[6] Reavis KM, McMillan G, Austin D, Gallun F, Fausti SA, et al. Distortion product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear. 2011; 32(1):61–74.
[7] Rybak LP, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Semin Hear. 2019; 40(2):197–204.
[8] Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004; 25(6):910–915.
[9] Waters GS, Ahmad M, Katsarkas A, Stanimir G, McKay J. Ototoxicity due to cis-diamminedichloroplatinum in the treatment of ovarian cancer: influence of dosage and schedule of administration. Ear Hear. 1991; 12(2):91–102.
[10] Kim MB, Zhang Y, Chang Y, Ryu S, Choi Y, et al. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017; 46(2):717–726.
[11] Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981; 23(7):493–500.
[12] Tserga E, Nandwani T, Edvall NK, Bulla J, Patel P, et al. The genetic vulnerability to cisplatin ototoxicity: a systematic review. Sci Rep. 2019; 9(1):3455.
[13] Arora R, Thakur JS, Azad RK, Mohindroo NK, Sharma DR, et al. Cisplatin based chemotherapy: Add high frequency audiometry in the regimen. Indian J Cancer. 2009; 46:311s–7.
[14] Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998; 77(8):1355–1362.
[15] Schmidt CM, Knief A, Lagosch AK, Deuster D, Zehnhoff-Dinnesen A. Left-right asymmetry in hearing loss following cisplatin therapy in children—the left ear is slightly but significantly more affected. Ear Hear. 2008; 29(6):830–837.
[16] Dutta A, Venkatesh MD, Kashyap RC. Study of the effects of chemotherapy on auditory function. Indian J Otolaryngol Head Neck Surg. 2005; 57(3):226–228.
[17] Daldal A, Odabasi O, Serbetcioglu B. The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg. 2007; 137(5):747–752.
[18] Haugnes HS, Stenklev NC, Brydøy M, Dahl O, Wilsgaard T, et al. Hearing loss before and after cisplatin-based chemotherapy in testicular cancer survivors: a longitudinal study. ActaOncol. 2018; 57(8):1–9.
[19] Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol. 2016; 34(23):2712–2720.
[20] Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and southwest oncology group study. J Clin Oncol. 1999; 17(5):1339–1339.
[21] Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999; 340(15):1137–1143.
[22] Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340(15):1144–1153.
[23] Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004; 22(5):872–880.
[24] Peters III WA. Cisplatin and 5-fluorouracil plus radiation therapy are superior to radiation therapy as adjunctive in high-risk early stage carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: Report of a phase III intergroup study. Gynecol Oncol. 1999; 72:443.
[25] Green J, Kirwan J, Tierney J, Vale C, Symonds P, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005; (3):CD002225.
[26] Vale C, Tierney JF, Stewart LA, Brady M, Dinshaw K, et al. Chemoradiotherapy for cervical cancer meta-analysis collaboration reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008; 26(35):5802–5812.
[27] Nandakumar A, Kishor Rath G, Chandra Kataki A, Poonamalle Bapsy P, Gupta PC, et al. Concurrent chemoradiation for cancer of the cervix: Results of a multi-institutional study from the setting of a developing country (India). J Glob Oncol. 2015; 1(1):11–22.