Orginal Research
2022
September
Volume : 10
Issue : 3
Prevalence of retinopathy in newly diagnosed type 2 diabetes mellitus patients and its association with microalbuminuria and HbA1c: A cross sectional study from Rajasthan
Praveena T, Kumar A, Arya RK
Pdf Page Numbers :- 164-168
Praveena T1, Ashok Kumar2,*, and Arya RK3
1Department of Ophthalmology, JLN Medical College, Ajmer, Rajasthan-305001, India
2Department of Community Medicine, JLN Medical College, Ajmer, Rajasthan-305001, India 3Department of General Medicine, All India Institute of Medical Sciences, Jodhpur, Rajasthan 342005, India
*Corresponding author: Dr. Ashok Kumar, Senior Demonstrator, Department of Community Medicine, JLN Medical College, Ajmer, Rajasthan-305001, India. Email: ashuchaniya@gmail.com
Received 28 April 2022; Revised 3 June 2022; Accepted 11 June 2022; Published 20 June 2022
Citation: Praveena T, Kumar A, Arya RK. Prevalence of retinopathy in newly diagnosed type 2 diabetes mellitus patients and its association with microalbuminuria and HbA1c: A cross sectional study from Rajasthan. J Med Sci Res. 2022; 10(3):164-168. DOI: http://dx.doi.org/10.17727/JMSR.2022/10-30
Copyright: © 2022 Praveena T et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Diabetes mellitus is a major public health problem with multiple medical complications. Microvascular complications of diabetes including diabetic retinopathy, depend on the duration and severity of hyperglycemia. There is a paucity of data about the relationship among diabetic retinopathy, microalbuminuria and HbA1c in newly diagnosed type 2 diabetes mellitus (T2DM) patients. The study was conducted on the prevalence of diabetic retinopathy and its association with microalbuminuria and HbA1c among newly diagnosed T2DM patients.
Material and method: It was a hospital-based, cross-sectional study conducted at a tertiary care hospital in Rajasthan. After obtaining written informed consent, data were collected from 150 newly diagnosed T2DM patients. Data were analysed using SPSS 20.0. Categorical variables were presented as proportion and continuous variables were presented as mean (SD). Chi square test and one way ANOVA tests were used for bivariate analysis.
Results: The mean (SD) age of patients was 50.43 (12.73) years. About 52.7% (n=79) patients were male. About 30% (n=45) of newly diagnosed T2DM patients had microalbuminuria. Thirty patients (20%) had diabetic retinopathy (DR). About 8% (n=12) participants had mild DR, 10.7% (n=16) had moderate to severe DR and 1.3 (n=2) % had proliferative DR. Microalbuminuria was found significantly associated with HbA1c (P<0.01). Diabetic retinopathy and its severity were also found significantly associated with HbA1c (P<0.01).
Conclusion: In newly diagnosed T2DM patients, HbA1c and microalbuminuria are associated with the presence of retinopathy. If follow up studies support these results, periodic ophthalmologic monitoring may be beneficial for those newly diagnosed with T2DM.
Keywords: retinopathy; diabetes; microalbuminuria; HbA1c
Full Text
Introduction
Diabetes mellitus (DM) has long been recognized as a major public health problem with far-reaching consequences, not only for its adverse health impact on individuals but also for its economic burden on the health care system [1, 2].
People with diabetes are vulnerable to multiple medical complications. These complications involve both macrovascular disease (heart disease, stroke) and microvascular disease [2]. The major microvascular complications are diabetic retinopathy, nephropathy, and neuropathy [3]. Diabetic retinopathy is the most common microvascular complication of diabetes [4, 5]. The risk of developing diabetic retinopathy or other microvascular complications of diabetes depends on the duration and severity of hyperglycemia. Diabetic retinopathy may begin to develop as early as 7 years before the diagnosis of diabetes in patients with type 2 diabetes [5]. Advanced glycation end products (including glycosylated haemoglobin, HbA1c) are known to produce micro-vascular complications in diabetic retinopathy [6-8]. Higher amounts of HbA1c in diabetic patients, indicating poorer control of blood glucose levels, have been associated with diabetic complications like; cardiovascular disease, nephropathy, and retinopathy [6, 7].
The concordance of microalbuminuria and diabetic retinopathy is well studied in type 2 diabetes; however, for newly diagnosed type 2 diabetes mellitus (T2DM), there is a paucity of data. The current study aimed to find out the prevalence of diabetic retinopathy and its association with microalbuminuria and HbA1c among newly diagnosed T2DM patients.
Material and methods
This hospital-based cross-sectional study was conducted from November 2020 to December 2021 in a tertiary care hospital in Rajasthan. Newly diagnosed T2DM patients were included in this study. Exclusion criteria were patients with acute or chronic renal failure, having dense cataract or corneal opacities, having coexisting ocular disorders likely to mask the findings of diabetic retinopathy, and diabetes with prior hypertension. The Ethics approval was obtained from the Institutional Review Board of the author’s institution. Written informed consent was taken from each participant.
Sample size: The sample size was calculated using a 16.5% prevalence of diabetic retinopathy in newly diagnosed T2DM patients in the available literature [9]. Estimated sample size was 147 with 6% absolute error and 95% confidence level, which was rounded off to 150. (Epi infoTM 7.2.5.0).
Sampling technique: Patients were enrolled from OPD. Consecutive sampling technique was adopted for data collection. DM was defined as a fasting plasma glucose of more than or equal to 126 mg/dl or a 2-hour plasma glucose level after a glucose tolerance test of more than or equal to 200 mg/dl or random plasma glucose of more than or equal to 200 mg/dl in the presence of symptoms of hyperglycemia i.e., polyuria, polydipsia, polyphagia and unexplained weight loss [10].
Data collection
Data were collected using an interviewer-administered, semi-structured, pre-tested questionnaire. It consisted of sociodemographic detail, plasma glucose measurements, urinary albumin estimation and fundus examination. Patients were categorized into three categories based on their HbA1c values. These were- good control (HbA1c <6.5%), fair control (HbA1c 6.5- 7.9%), poor control (HbA1c ≥8.0%) [10].
Urinary albumin level was measured with the Esbach test. Microalbuminuria was defined as albumin excretion of 30-299 mg/24 hours [11]. Severity of DR was clinically graded using Early Treatment Diabetic Retinopathy Study scales by a trained ophthalmologist. Diabetic retinopathy was recorded in four categories viz.- mild, moderate, severe and proliferative diabetic retinopathy [12].
Data management
Data were entered into a computer-based spreadsheet and cleaned. Data were analysed using SPSS 20.0. Categorical variables such as gender, microalbuminuria, diabetic retinopathy etc. were presented as proportion. Continuous variables such as age, HbA1c etc. were presented as mean (SD). Bivariate analysis was done with Chi-square test. P value <0.05 was considered significant.
Results
One hundred sixty-seven newly diagnosed T2DM patients were approached for the study. Seven refused to give consent, and 10 patients couldn’t give complete information related to the study (Response rate= 89.8%). Data were analysed from 150 patients. The mean (SD) age of patients was 50.43 (12.73) years. About 52.7% (n=79) patients were male. The mean (BMI) of the patients was 25.43 (4.97) Kg/m2. About 55.3% (n=83) patients had normal BMI. About 4.7% (n=7) had BMI <18.5 Kg/m2, 22% (n=33) had BMI from 25.01 to 30.0 Kg/m2, and 18% (n=27) had BMI >30 Kg/m2 (Table 1).
Table 1: Distribution of participants according to age, gender and BMI (n=150).
|
|
Number (n)
|
Per cent (%)
|
|
Age (Years)
|
|
|
<30
|
9
|
6.0
|
|
|
31- 50
|
68
|
45.33
|
|
|
51-70
|
70
|
46.67
|
|
|
>70
|
3
|
2.0
|
|
|
Mean (SD)
|
50.43 (12.73)
|
|
Gender
|
|
|
Male
|
79
|
52.7
|
|
|
Female
|
71
|
47.3
|
|
BMI (Kg/m2)
|
|
|
<18.5
|
7
|
4.7
|
|
|
18.5- 25
|
83
|
55.3
|
|
|
25.01- 30
|
33
|
22.0
|
|
|
>30
|
27
|
18.0
|
|
|
Mean (SD)
|
25.43 (4.97)
|
About 30% (n=45) of newly diagnosed T2DM patients had microalbuminuria. Thirty patients (20%) had diabetic retinopathy. Microalbuminuria was found significantly associated with HbA1c (P<0.01). The presence of DR was also found significantly associated with HbA1c (P<0.01) (Table 2 & Figure 1).
Table 2: Association of HbA1c with microalbuminuria and diabetic retinopathy among newly diagnosed diabetic patients (n= 150).
|
|
Total
|
HbA1c
|
P value
|
|
Good
(n= 45)
N (%)
|
Fair
(n= 57)
N (%)
|
Poor
(n= 48)
N (%)
|
|
Micro-albuminuria
|
Present
|
45
|
4 (8.9)
|
13 (22.8)
|
28 (58.3)
|
<0.01*
|
|
Absent
|
105
|
41 (91.1)
|
44 (77.2)
|
20 (41.7)
|
|
Diabetic retinopathy
|
Present
|
20
|
3 (6.7)
|
9 (15.8)
|
18 (37.5)
|
<0.01*
|
|
Absent
|
130
|
42 (93.3)
|
48 (84.2)
|
30 (62.5)
|
*Statistically significant.
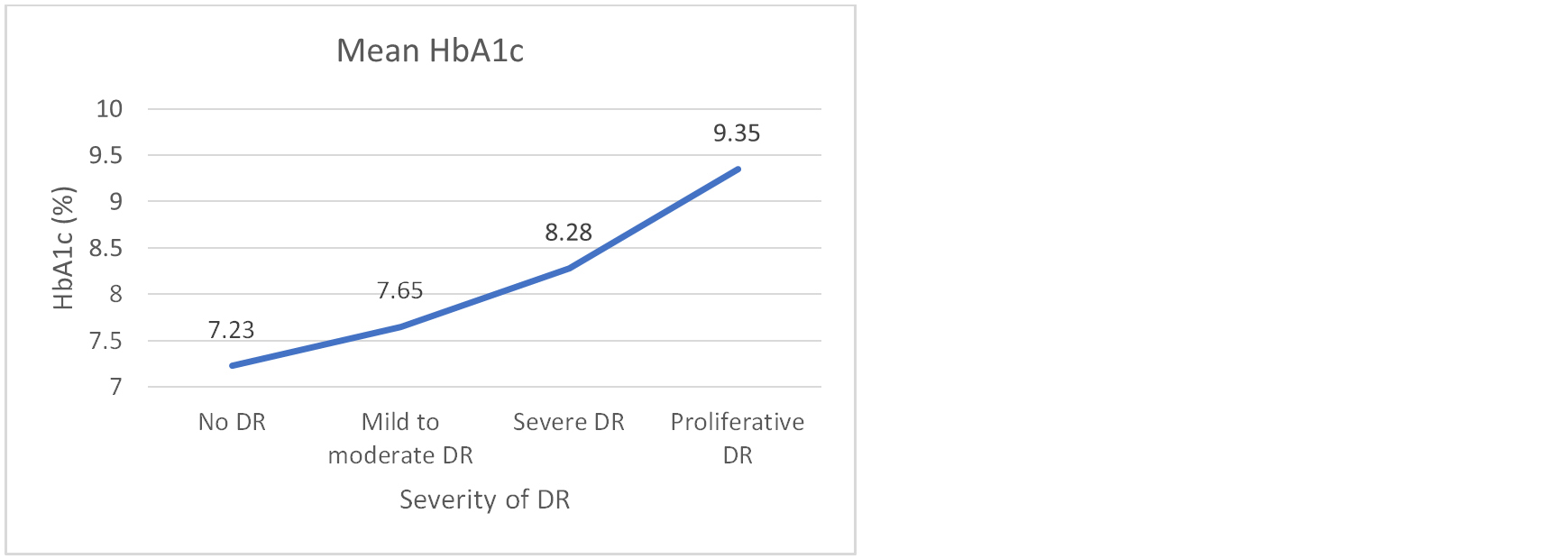
Figure 1: Association of mean HbA1c with severity of DR (n=150). One way ANOVA test, F statistics = 3.81, P= 0.011*. *Statistically significant.
About 8% (n=12) participants had mild DR, 10.7% (n=16) had moderate to severe DR and 1.3 (n=2) % had proliferative DR. Severity of DR was found significantly associated with HbA1c (P<0.01). Severity of DR was also found associated with presence of microalbuminuria (P<0.01) (Table 3).
Table 3: Association of severity of diabetic retinopathy with HbA1c and microalbuminuria (n=150).
|
|
Total
|
No DR
(n=120)
N (%)
|
Mild
(n=12)
N (%)
|
Moderate to severe
(n= 16)
N (%)
|
Proliferative
(n=2)
N (%)
|
P value
|
|
HbA1c
|
|
Good (<6.5 %)
|
45
|
42 (93.3)
|
2 (4.4)
|
1 (2.2)
|
0 (0)
|
<0.01*
|
|
Fair (6.5- 7.9 %)
|
57
|
48 (84.2)
|
3 (5.3)
|
6 (10.5)
|
0 (0)
|
|
Poor (≥8 %)
|
48
|
30 (62.5)
|
7 (14.6)
|
9 (18.8)
|
2 (4.2)
|
|
Microalbuminuria
|
|
Present
|
45
|
21 (46.7)
|
9 (20.0)
|
13 (28.9)
|
2 (4.4)
|
<0.01*
|
|
Absent
|
105
|
99 (94.3)
|
3 (2.9)
|
3 (2.9)
|
0 (0)
|
*Statistically significant.
Table 4 shows association between diabetic retinopathy and microalbuminuria across the three HbA1c categories. DR was associated with microalbuminuria across the three categories. Among good glycaemic control patients, odds of having DR were 40 times higher among patients with microalbuminuria than patients without microalbuminuria (OR= 40.0, 95% CI= 2.45- 65.34). Among fair glycaemic control patients, odds of having DR were 11.71 times higher among patients with microalbuminuria than patients without microalbuminuria (OR= 11.71, 95% CI= 2.36- 58.08). Among poor glycaemic control patients, odds of having DR were 12 times higher among patients with microalbuminuria than patients without microalbuminuria (OR= 12.0, 95% CI= 2.32- 61.95).
Table 4: Association of diabetic retinopathy and microalbuminuria across the three HbA1c categories (n= 150).
|
Glycaemic control
|
MA
|
Number
(n=150)
|
Diabetic retinopathy
|
OR
(95% CI)
|
P value
|
|
Present
(n= 30)
N (%)
|
Absent
(n=120)
N (%)
|
|
Good (<6.5 %)
|
Present
|
4
|
2 (50.0)
|
2 (50.0)
|
40.0
(2.45- 65.34)
|
<0.01
|
|
Absent
|
41
|
1 (2.4)
|
40 (97.6)
|
|
Fair (6.5- 7.9 %)
|
Present
|
13
|
6 (46.2)
|
7 (53.8)
|
11.71
(2.36- 58.08)
|
0.01
|
|
Absent
|
44
|
3 (6.8)
|
41 (93.2)
|
|
Poor (≥8 %)
|
Present
|
28
|
16 (57.1)
|
12 (42.9)
|
12.0
(2.32- 61.95)
|
0.01
|
|
Absent
|
20
|
2 (10.0)
|
18 (90.0)
|
Discussion
One hundred fifty newly diagnosed T2DM patients were included in the study. The mean (SD) age of the participants was 50.43 (12.73) years. In the present study, the prevalence of microalbuminuria among newly diagnosed T2DM patients was found 30%. Similar prevalence (32.9) was also reported in North India by Mir et al [13]; whereas, in Nigeria, it was reported higher i.e. prevalence rate of 50% [14]. The variation in the prevalence of microalbuminuria can be attributed to factors such as different methods of estimation of microalbuminuria, ethnic differences in the study populations, definitions of microalbuminuria, and adopted methods of urine collection, etc.
In the current study, microalbuminuria was found associated with HbA1c. There was a steady increase in the prevalence of microalbuminuria with increasing HbA1c. Similar findings were also reported in North India by Mir et al [13]. United Kingdom Prospective Diabetes Study (UKPDS) study also showed that microvascular complications benefit from better control of blood glucose levels [15]. In a study on newly diagnosed T2DM patients in Nigeria, the authors also observed that an HbA1c value above 8% was associated with a higher incidence of microalbuminuria [16].
The prevalence of diabetic retinopathy was found as 20% in the current study. This was consistent with studies done in United Kingdom by Chowdhury et al [9] and Shah et al [17] where they found a 16.5% and 18% prevalence of DR, respectively. In India, Garg et al also found a similar prevalence i.e. 22.1% of diabetic retinopathy among T2DM patients living with diabetes for less than 10 years [18]. Whereas, Rani et al and Reddy et al observed a higher prevalence of diabetic retinopathy i.e. 31% and 36.5% respectively, in their studies conducted in India [19, 20]. In North India, Narang et al found 45% prevalence of diabetic retinopathy [21]. The risk of retinopathy in T2DM increases with the duration of diabetes. Only newly diagnosed T2DM patients were included in this study, which may be the reason for the comparatively lower prevalence of diabetic retinopathy in this study.
Diabetic retinopathy was found to be associated with HbA1c. It was found more among patients with higher HbA1c. A study from Central India also reported that patients having a good glycemic control had a lower prevalence of diabetic retinopathy as compared to those having poor control [18]. In the present study, eight per cent of participants had mild DR, 10.7% had moderate to severe DR and 1.3% had proliferative DR. Garg et al also made similar observations i.e. 14.4% of participants had mild DR, 15.3% had moderate to severe DR and 2.4 % had proliferative DR in patients with <10 years duration of T2DM in their study from Central India [18]. In different cross-sectional studies, the prevalence of different grades of retinopathy was found of similar order with a prevalence of lower grades of retinopathy being higher compared to higher grades or proliferative retinopathy [20-22].
In the current study, it was also found that diabetic retinopathy was associated with microalbuminuria. Garg et al, Manaviat et al and Boelter et al also reported a significant linear relationship between microalbuminuria and the severity of diabetic retinopathy [18, 24, 25]. Few studies have identified that the renal changes seen in individuals with both microalbuminuria and retinopathy had a distinct pattern compared to those having microalbuminuria without retinopathy [26].
The current study provides a deep insight into the relationship among microalbuminuria, diabetic retinopathy and HbA1c level among newly diagnosed T2DM. It was observed that higher levels of HbA1c and the presence of microalbuminuria, are associated with the occurrence of diabetic retinopathy. These are also associated with the severity of diabetic retinopathy.
Limitations
It was a cross-sectional study, so association doesn’t imply causation. The role of confounders was also not studied.
Conclusion
In newly diagnosed T2DM patients, HbA1c and microalbuminuria are associated with the presence of retinopathy. These findings imply that HbA1c and microalbuminuria may serve as predictors of the likelihood that proliferative retinopathy may occur. If follow up studies support these results, periodic ophthalmologic monitoring may be beneficial for newly diagnosed T2DM patients with high HbA1c and microalbuminuria. Acknowledgement Dr. Naudibya Majhi for her valuable feedback in editing of the manuscript.
Conflict of interest
Authors declare no conflict of interest.
References
[1] World Health Organisation. Diabetes: Fact sheet. World Health Organization, Geneva. Accessed on 5 Feb 2022 from: https://www.who.int/news-room/fact-sheets/detail/diabetes
[2] International Diabetes Federation. IDF Diabetes Atlas: Tenth Edition [Internet]. Accessed on 5 Feb 2022 from: https://diabetesatlas.org/
[3] Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes 2017. J Diabetes Res. 2018; 2018:e3086167.
[4] Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008; 88(11):1254–1264.
[5] Fong DS, Aiello LP, Ferris FL, Klein R. Diabetic retinopathy. Diabetes Care. 2004; 27(10):2540–2553.
[6] Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012; 55(3):636–643.
[7] Su JB, Zhao LH, Zhang XL, Cai HL, Huang HY, et al. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018; 17(1):1–9.
[8] Lind M, Odén A, Fahlén M, Eliasson B. The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLoS ONE. 2009; 4(2):e4412.
[9] Chowdhury SR, Thomas RL, Dunseath GJ, Peter R, Rees DA, et al. Diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus: contribution of β-Cell function. J Clin Endocrinol Metabol. 2016; 101(2):572–580.
[10] American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010; 33(Suppl 1):S62–S69.
[11] Toto RD. Microalbuminuria: Definition, Detection, and Clinical Significance. J Clin Hypertens. 2004; 6(Suppl 11):2–7.
[12] Solomon SD, Goldberg MF. ETDRS grading of diabetic retinopathy: Still the gold standard? Ophthalmic Res. 2019; 62(4):190–195.
[13] Mir SR, Bhat MH, Misgar RA, Mir IB, Wani AI, et al. Prevalence of microalbuminuria in newly diagnosed T2DM patients attending a tertiary care hospital in North India and its association with various risk factors. 2019; 6(4):9–13.
[14] Unuigbe E, Omeife H, Edema T, Ukoli F. Microalbuminuria and associated factors in newly diagnosed diabetics. Niger Postgrad Med J. 2001; 8:187–92.
[15] Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003; 63(1):225–232.
[16] Agaba E, Agaba P, Puepet FH. Prevalence of microalbuminuria in newly diagnosed type 2 diabetic patients in Jos Nigeria. Afr J Med Sci. 2004; 33:19–22.
[17] Shah S, Feher M, McGovern A, Sherlock J, Whyte MB, et al. Diabetic retinopathy in newly diagnosed Type 2 diabetes mellitus: Prevalence and predictors of progression; a national primary network study. Diabetes Res Clin Pract. 2021; 175:e108776.
[18] Garg P, Misra S, Yadav S, Singh L. Correlative study of diabetic retinopathy with HbA1c and microalbuminuria. Int J Ophthalmic Res. 2018; 4(2):282–286.
[19] Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, et al. Albuminuria and diabetic retinopathy in type 2 diabetes mellitus Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study. Diabetol Metab Syndr. 2011; 3(1):9.
[20] Reddy SC, Kihn YM, Nurjahan MI, Ramil A. Retinopathy in type 2 diabetic patients with microalbuminuria. Nepal J Ophthalmol. 2013; 5(1):69–74.
[21] Narang U, Jagadhami V, Singla M, Singal KK, Agarwal R, et al. A study of prevalence of microalbuminuria and diabetic retinopathy in rural patients presenting to a tertiary care hospital in North India. Int J Contem Med. 2019; 6(8):18–22.
[22] Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008; 4(4):216–26.
[23] Ichinose K, Kawasaki E, Eguchi K. Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol. 2007; 27(6):554–564.
[24] Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmology. 2004; 4(1):1–4.
[25] Boelter MC, Gross JL, Canani LH, Costa LA, Lisboa HR, et al. Proliferative diabetic retinopathy is associated with microalbuminuria in patients with type 2 diabetes. Braz J Med Biol Res. 2006; 39(8):1033–1039.
[26] Olivarius NDF, Nielsen NV, Andreasen AH. Diabetic retinopathy in newly diagnosed middle-aged and elderly diabetic patients. Prevalence and interrelationship with microalbuminuria and triglycerides. Graefes Arch Clin Exp Ophthalmol. 2001; 239(9):664–672.