Full Text
Introduction
Abnormal uterine bleeding (AUB) is one of the common disorders for which women seek gynecological consultation. It affects at least one–fourth women in the reproductive age group [1] often affecting their quality of life. It is defined [2] as any variation from a normal menstrual cycle i.e., a frequency more than or less than 24 to 38 days, lasting more than 7 to 9 days and blood loss exceeding 80mL. In 2011 FIGO had devised a new classification (PALM-COEIN) [3] for categorizing AUB into 9 main categories of abnormal uterine bleeding: polyp; adenomyosis; leiomyoma; malignancy and hyperplasia; coagulopathy; ovulatory dysfunction; endometrial; iatrogenic; and not yet classified. The purpose of the classification was to limit the requirement of surgery as the primary modality of treatment for all cases of AUB. These pathologies can be diagnosed by various techniques like trans- abdominal and trans-vaginal ultrasound, saline infusion sonography, hysteroscopy and by endometrial biopsy and histopathology [4]. Currently transabdominal and transvaginal ultrasound are the commonly used primary screening modality [5] whereas uterine dilatation and uterine curettage [D&C] is the frequently used technique in diagnosis of abnormal uterine bleeding. However these methods have their own shortcomings. Ultrasound cannot effectively differentiate between a polyp and hyperplastic endometrium [6] and may sometimes miss out on focal endometrial changes. D&C is a blind procedure with chances of missing focal lesions during biopsy [7]. Currently, diagnostic hysteroscopy and guided biopsy is gradually emerging as a gold standard in the diagnosis and management of AUB. The objective of our current study was to evaluate the role of hysteroscopy in diagnosis and management of AUB.
Materials and methods
This study was retrospective hospital record based study done at Shri Shankaracharya Institute of Medical Sciences, Bhilai a tertiary care medical college hospital for a period of one year from January to December 2020. It was a retrospective observational study. Inclusive sampling was done. In total there were 177 patients above 18 years of age who had undergone diagnostic hysteroscopy for abnormal uterine bleeding during the study period. Among the patients who underwent hysteroscopy, in four patients with atrophic endometrium no biopsy was obtained and one patient had a retained Cu-T. These patients were excluded and the records of 172 were included in the study. In each of these patients, hysteroscopy was done under total intravenous anesthesia, normal saline was used as distension medium and endometrial tissue was sent for histopathological evaluation. After the procedure patients were discharged on the same evening and were asked to follow up in the OPD after a week for further management based on the histopathology reports. Institutional ethics clearance was taken before the commencement of the study. The flow of study is given in Figure 1.
Figure 1: Flow of study.
The hysteroscopic diagnosis was compared to final histopathology report, sensitivity, specificity, positive predictive value of hysteroscopy for diagnosis of various intrauterine pathology was calculated. Statistical analysis was done with MedCalc statistical software.
Results
In our study majority of women fell in age group of 41 to 50 years (n=89, 52%) (Table 1). Most patients (n=76, 44%) already had a history of bleeding for more than a year before they sought medical attention and most of the patients (n=97, 56%) had heavy menstrual bleeding (Table 2).
Table 1: Age distribution of patients.
|
Age
|
Number of patients (N=172)
|
Percentage %
|
|
20-30
|
9
|
5
|
|
31 -40
|
45
|
26
|
|
41-50
|
89
|
52
|
|
≥51 yrs
|
29
|
17
|
Table 2: Duration and distribution of patient symptoms.
|
Duration
|
Number of patients ( N=172)
|
Percentage %
|
|
|
<6 months
|
38
|
22
|
|
|
6-12 months
|
58
|
34
|
|
|
>1 year
|
76
|
44
|
|
|
Distribution of patients symptoms
|
|
Heavy menstrual bleeding
|
97
|
56.4
|
|
Intermenstrual bleeding
|
57
|
33.1
|
|
Postmenopausal bleeding
|
18
|
10.
|
On hysteroscopy 37 patients had secretory endometrium (pink, smooth thickened in few cases white glandular opening could be seen) and on histology 35 of these patients were confirmed with secretory endometrium whereas 2 patients were diagnosed as proliferative endometrium.
62 patients appeared to have proliferative endometrium on hysteroscopy (pink smooth and thin) out of which 34 patients had proliferative endometrium even on histopathology.
Atrophic endometrium (Figure 2a) appeared as pale with scattered interspersed areas of petechiae and haemorrhage on hysteroscopy 14 patients had atrophic endometrium of which all were reported as atrophic on histopathology.
Hyperplasia (Figure 2b) appeared thickened, edematous, undulating on hysteroscopy 16 patients had hyperplastic endometrium on hysteroscopy and 14 of these patients had hyperplasia on their histopathology reports. Polyps (Figure 2c) appeared as pink tongue like projection on hysteroscopy. In 24 patients we found polypoid growth which was excised and send for HPE of which we found 17 patients turned out to have polyp on HPE and 2 turned out to be hyperplasia and 4 turned out to have disordered proliferation and 1 turned out to be fibroid polyp. Fibroids (Figure 2d) or leiomyoma appeared as indentations in the uterine cavity. On taking biopsy from these lesions we found 16 patients to have histopathology also reported as same and no patients were missed with submucous fibroid.
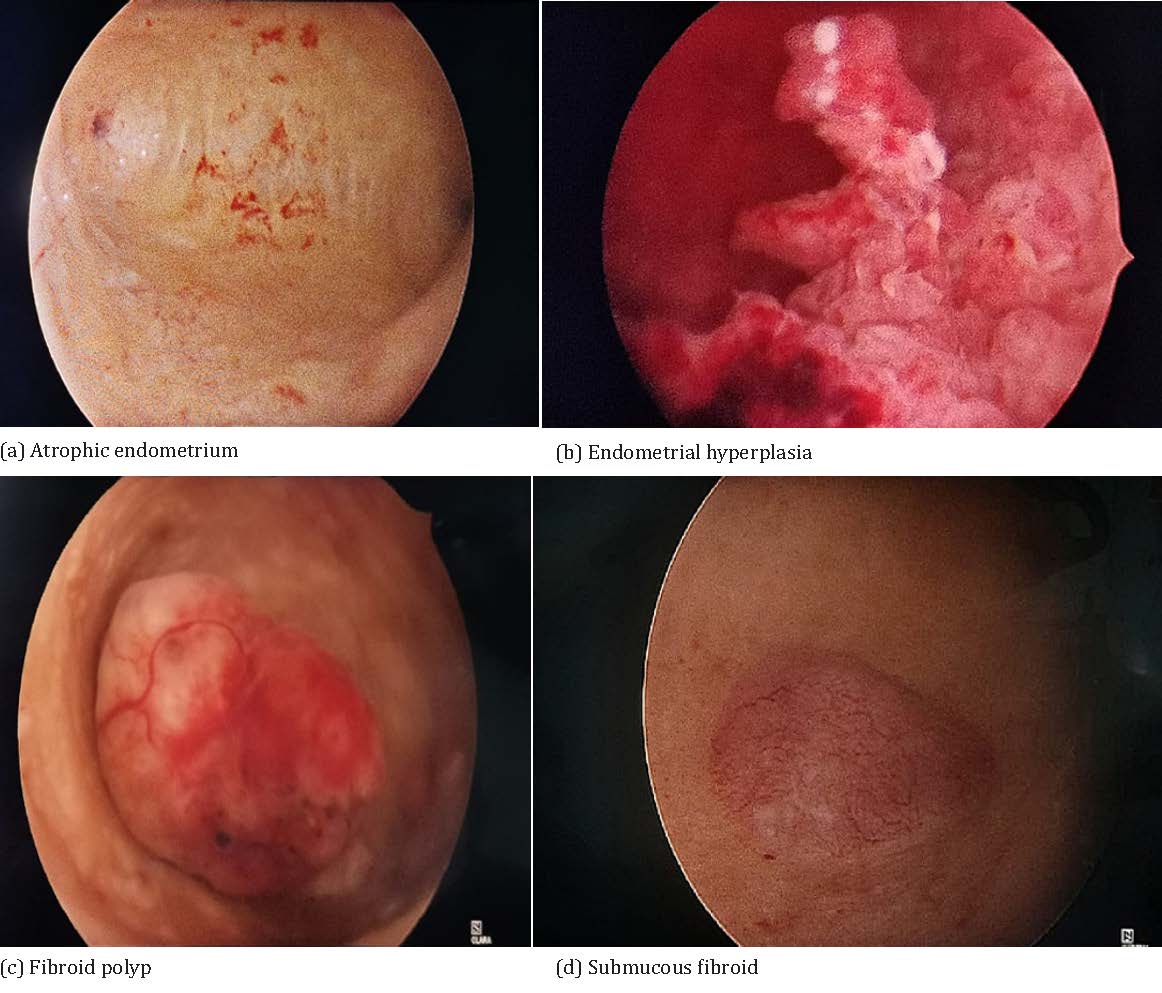
Figure 2: Hysteroscopic appearance of various pathologies.
In one patient on hysteroscopy found distorted endometrial cavity with focal areas of necrosis and atypical vessels which was suspicious of carcinoma. It was subsequently confirmed on histopathology.
Two patients were suspected of having retained products on hysteroscopy and both of them turned out as the same on histopathology. In sixteen patients, histopathology was reported as “disordered proliferative” which were mostly diagnosed as proliferative endometrium on hysteroscopy.
The frequency of various hysteroscopic diagnosis, the number of diagnoses found to be correct on histopathology, the overall reported histopathology and the sensitivity, specificity and positive predictive value of hysteroscopy for diagnosing these conditions can be found on Table 3.
Table 3: Co-relation between hysteroscopic diagnosis and final histopathology.
|
Pathology
|
Hysteroscopic finding (n=)(%)
|
Out of which confirmed on histopathology (n=)
|
Overall reported histopathology finding (n=)(%)
|
Sensitivity (%)
|
Specificity (%)
|
Positive predictive value (%)
|
|
Secretory endometrium
|
37 (21.5)
|
35
|
51
|
71.43
|
98.37
|
94.59
|
|
Proliferative endometrium
|
62 (36)
|
34
|
38
|
100.00
|
79.71
|
54.84
|
|
Endometrial polyp
|
24(13.95)
|
17
|
17
|
100.00
|
95.68
|
70.83
|
|
Leiomyoma
(submucous fibroid)
|
16(9.3)
|
16
|
16
|
100.00
|
100.00
|
100.00
|
|
Carcinoma endometrium
|
01(0.58)
|
01
|
01
|
100.00
|
100.00
|
100.00
|
|
Simple and atypical hyperplasia
|
16(9.3)
|
14
|
17
|
82.35
|
98.71
|
87.50
|
|
Atrophic Endometrium
|
14(8.1)
|
14
|
14
|
100.00
|
100.00
|
100.00
|
|
RPOC
|
02(1.1)
|
02
|
02
|
100.00
|
100.00
|
100.00
|
|
Disordered proliferative
|
0
|
0
|
16
|
--
|
--
|
--
|
|
Total
|
172
|
--
|
172
|
--
|
--
|
--
|
Discussion
AUB is a common gynaecological problem and its incidence is around 30-40%[7]. The commonly performed diagnostic and therapeutic procedure in such cases is dilatation and curettage (D&C). D&C is a blind procedure and can miss more than half of endometrial lesions when done alone, in cases of atrophic endometrium it is also sometimes difficult to obtain biopsy or no biopsy can be obtained. For AUB, D&C also has quite poor sensitivity and overall diagnostic accuracy [8], and has been regarded as inadequate diagnostic and therapeutic tool for uterine disorders by some [9].
All of these disadvantages of D&C are avoided in hysteroscopy. Hysteroscopy is a very simple and low risk procedure. Unlike D&C, it allows direct visualisation under good illumination and magnified vision permitting adequate exploration of the uterine cavity. It helps in prompt diagnosis of several conditions with very low complication rates. Few large national level studies have reported a complication rate of less than one percent (n=24191) [10].
One definite and major advantage of hysteroscopy is that direct visualisation of the lesion in most cases helps readily to arrive at the correct diagnosis without having to wait for histopathology, which helps to either start empirical treatment promptly or classify patients as high risk for malignancy and accelerate their management. This advantage is totally unavailable in a blind procedure like D&C where treatment can be instituted only after receiving the final histopathology report which takes at least a week or more.
Hysteroscopy has several therapeutic advantages too. In case of endometrial polyp and pedunculated leiomyoma, diagnosis and treatment can be done in the same sitting. Panoramic vision of the entire endometrium enables detection of focal areas of abnormality and directed biopsies can be taken from those areas with ease.
Diagnosis of atrophic endometrium is best done by hysteroscopy and it is very reassuring for the patients with post-menopausal bleeding. Many times blind curettage does not yield any endometrium for the diagnosis to be made. Sometimes if any suspicious lesion is present, it can be easily detected in the background of atrophic endometrium, so directed biopsy can exclude endometrial carcinoma. Tinelli et al recommends that hysteroscopy should be done in all cases of AUB with a thin endometrium (<4mm) [11].
Hysteroscopy also allows for retrieval of misplaced IUCDs after detection on ultrasound.
The current study found that hysteroscopy has 100% sensitivity in detection of proliferative endometrium,endometrial polyp, leiomyoma (submucous fibroid), atrophic endometrium and retained products of conception. It also diagnosed carcinoma endometrium correctly, although number of such diagnoses were few.
Although sensitivity was relatively lower for detecting secretory endometrium (71.43) and hyperplasia (82.35), but it was very specific (98.37 and 98.71 respectively) with a high positive predictive value.
According to a study done by Pasquale et al [12] the sensitivity of diagnostic hysteroscopy followed by endometrial biopsy for detection of endometrial hyperplasia is 90.4 % which is very similar to our findings (sensitivity for detection of endometrial hyperplasia - 82.35%) however in our study specificity was higher (98% vs 48.9%).
In a similar study done by Patil et al [13], they found that in detection of hyperplasia is sensitivity was around 75%, specificity 92 which compares favourably with our findings (sensitivity of 81.2% and specificity of 98 %). For detection of polyp the sensitivity and specificity are also similar in both the studies. For submucous fibroid they reported a sensitivity of 100 % and specificity 89% whereas in our study sensitivity and specificity were both 100%. Junnare [14] in their study comparing hysteroscopy with transvaginal sonography, found that hysteroscopy has a high sensitivity and specificity for detecting endometrial pathology like proliferative, secretory, atrophic endometrium as well as hyperplasia and polyp. A study by Atunnes [15] also had similar results in which they grouped the pathologies into uterine cavity lesions and endometrial aspect lesions. The comparison of sensitivity and specificity of hysteroscopy in diagnosing various pathologies in various studies can be compared in Table 4.
Table 4: Comparison with other studies.
|
|
Current study (n=172)
|
Patil et al ( 2009) (n=100) [13]
|
K.K Junnare et al (2019) (n=98) [14]
|
Attunes A.A.R (2011) (n= 419) [15]
|
|
Pathology
|
Sensitivity
|
Specificity
|
Sensitivity
|
Specificity
|
Sensitivity
|
Specificity
|
Sensitivity
|
Specificity
|
|
Endometrial polyp
|
100%
|
95.68%
|
100%
|
95%
|
95.2%
(All pathology combined)
|
92.8%
(All pathology combined)
|
99.3% (cavity lesions)
79.8% (aspect lesions)
|
97.7% (cavity lesions)
91.7% (aspect lesions)
|
|
Hyperplasia
|
82.35%
|
98.71%
|
75%
|
92%
|
|
Submucous fibroid
|
100%
|
100%
|
100%
|
89%
|
|
Carcinoma endometrium
|
100%
|
100%
|
|
|
|
|
60%
|
99.5%
|
These results unequivocally show that hysteroscopy is a highly sensitive and specific diagnostic procedure for detection of almost all endometrial lesions and probably time has come to discard the older D&C technique in its favour. Hysteroscopy can be performed under the same total intravenous anesthesia like D&C and requires almost same time duration. One definite advantage of D&C is its simplicity in not requiring sophisticated equipment like hysteroscope, light source, monitor etc. and can be performed in most primary and rural setups if anesthetist is available. But gradually facilities for basic laparoscopy are becoming more and more available even in small setups and rural hospitals, thereby making hysteroscopy quite feasible.
The current study was an observational study. And as such one of the important limitations is the absence of any control group which would make the results more accurate and valid. Also sampling was inclusive and study period was relatively short thereby having all the disadvantages of not having a non-randomized study. The results will be more valid with a larger sample size and with a comparison group.
Conclusion
Although office hysteroscopy and “ambulatory” hysteroscopy [16] are quite prevalent in western countries, dilatation and uterine curettage for abnormal uterine bleeding continues to be quite popular in a country like India, which might be because of economic and other reasons. But with the increasing availability of laparoscopic facilities even in peripheral health setups, hysteroscopy has become increasingly feasible. Our study clearly demonstrates that hysteroscopy is a highly sensitive and specific diagnostic procedure for intrauterine lesions and has several advantages over a blind procedure like uterine curettage. Safety of hysteroscopy is also well established with minimal complication rates. Therefore the authors recommend using hysteroscopy over dilatation and curettage as the better procedure, both diagnostic and therapeutic for all cases of abnormal uterine bleeding and this technique with low complication rates is quite feasible to be used in most types of medical setups and must be popularized.
Informed consent
Informed consent for publication was taken from all subjects for utilizing clinical information and images and that every effort will be made to keep information anonymous.
Conflicts of interests
The authors declare no conflicts of interests.
References
[1] Whitaker L, Critchley H O. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016; 34:54–65.
[2] Davis E, Sparzak PB. Abnormal uterine bleeding (Updated 2022 Feb 10). StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532913.
[3] Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011; 113(1):3–13.
[4] Munro MG, Southern California Permanente Medical Group’s Abnormal Uterine Bleeding Working Group. Investigation of women with postmenopausal uterine bleeding: clinical practice recommendations. Perm J Winter. 2014; 18(1):55–70.
[5] ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018; 131(5):e124–e129.
[6] Breitkopf DM, Frederickson RA, Snyder RR. Detection of benign endometrial masses by endometrial stripe measurement in premenopausal women. Obstet Gynecol. 2004; 104(1):120–125.
[7] Kolhe S. Management of abnormal uterine bleeding - focus on ambulatory hysteroscopy. Int J Womens Health. 2018; 10:127–136.
[8] Yarandi F, Izadi-Mood N, Eftekhar Z, Shojaei H, Sarmadi S. Diagnostic accuracy of dilatation and curettage for abnormal uterine bleeding. J Obstet Gynaecol Res. 2010; 36(5):1049–1052.
[9] Bettocchi S, Ceci O, Vicino M, Marello F, Impedovo L, et al. Diagnostic inadequacy of dilatation and curettage. Fertil Steril. 2001; 75(4):803–805.
[10] Aas-Eng, MK, Langebrekke, A, Hudelist, G. Complications in operative hysteroscopy – is prevention possible? Acta Obstet Gynecol Scand. 2017; 96(12):1399–1403.
[11] Tinelli R, Tinelli FG, Cicinelli E, Malvasi A, Tinelli A. The role of hysteroscopy with eye-directed biopsy in postmenopausal women with uterine bleeding and endometrial atrophy. Menopause. 2008; 15(4 Pt 1):737–742.
[12] De Franciscis P, Riemma G, Schiattarella A, Cobellis L, Guadagno M, et al. Concordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics (Basel). 2019; 9(4):142.
[13] Patil SG, Bhute SB, Inamdar SA, Acharya NS, Shrivastava DS. Role of diagnostic hysteroscopy in abnormal uterine bleeding and its histopathologic correlation. J Gynecol Endosc Surg. 2009; 1(2):98–104.
[14] Junnare KK, Desai GJ, Shekhawat GS. Hysteroscopy: an effective tool in post-menopausal bleeding. IJRCOG. 2019; 8(1):159–164.
[15] Antunes AAR. The efficacy of hysteroscopy in diagnosis and treatment of endometrial pathology. Gynecol Surg. 2012; 9:47–52.
[16] Connor M. New technologies and innovations in hysteroscopy. Best Pract Res Clin Obstet Gynaecol. 2015; 29(7):951–965.