Orginal Research
2022
September
Volume : 10
Issue : 3
Etiological spectrum of cardioembolic strokes in a tertiary care hospital of India: Analysis of one year data
Gudapati MK, Sirisha S, Patil A, Manukinda J, Yada PK, Jayalakshmi S, Varalakshmi EA, Kaul S, Surath M
Pdf Page Numbers :- 118-122
Mahathi Krishna Gudapati1, Sai Sirisha1, Anuja Patil1, Jayasree Manukinda1, Praveen Kumar Yada1, Sita Jayalakshmi1, Varalakshmi EA1, Subhash Kaul1,*, and Mohandas Surath1
1Department of Neurology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. Subhash Kaul, Sr. Consultant Neurologist, Department of Neurology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Email: subashkaul@hotmail.com
Received 11 May 2022; Revised 13 June 2022; Accepted 21 June 2022; Published 30 June 2022
Citation: Gudapati MK, Sirisha S, Patil A, Manukinda J, Yada PK, Jayalakshmi S, Varalakshmi EA, Kaul S, Surath M. Etiological spectrum of cardioembolic strokes in a tertiary care hospital of India: Analysis of one year data. J Med Sci Res. 2022; 10(3):118-122. DOI: http://dx.doi.org/10.17727/JMSR.2022/10-22
Copyright: © 2022 Gudapati MK et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Traditionally rheumatic heart disease with mitral stenosis and atrial fibrillation has been the common mechanism of cardioembolic strokes in India. However, with the increase in life span and higher detection and development of cardiac diseases, the frequency and etiologic spectrum of cardioembolic stroke in an Indian hospital may be changing.
Methods: The case records of patients admitted in the year 2020 were analyzed for the presence of risk factors and identified stroke mechanisms.
Results: Among 538 patients of ischemic strokes, cardioembolic source was identified in 156 (29.8%) patients. Five major cardiac sources of embolism were identified: Coronary artery disease (with acute or remote myocardial infarction) 56/156 (35.89%), non valvular atrial fibrillation, 34/156(21.79%), post cardiac intervention 28/156 (17.94%), left atrial cardiomyopathy 20/156 (12.8%), and rheumatic heart disease 13/156 (8.3%).
Conclusion: Coronary artery disease, non valvular atrial fibrillation, post intervention stroke and left atrial cardiomyopathy are emerging as common causes of cardioembolic strokes. Rheumatic heart disease continues to be an important but less frequent cause of cardioembolic strokes.
Keywords: cardioembolic strokes; rheumatic heart disease; etiological spectrum; India
Full Text
Introduction
More than two-thirds of all acute strokes are ischemic in origin [1]. Cardioembolic strokes account for more than a quarter of all ischemic strokes [2]. Leading reported causes in India include rheumatic heart disease (RHD) and coronary artery disease (CAD) [3]. With the growth in life expectancy and increased use of newer investigations such as prolonged Holter monitoring, risk factors such as nonvalvular atrial fibrillation (NVAF) are being detected more frequently in recent years [4].
The aim of our study was to investigate the etiological spectrum of cardioembolic stroke in a tertiary care hospital in Hyderabad, a metropolitan city in India.
Methods
The case records of all the stroke patients admitted from January 1, 2020 to December 31, 2020 at the Krishna Institute of Medical Sciences (KIMS), a leading tertiary care referral center in Hyderabad were analyzed. All the patients of acute ischemic stroke were uniformly investigated to determine the underlying mechanism of stroke with magnetic resonance imaging and angiogram (MRI/MRA) of brain, carotid duplex examination, electrocardiography, two-dimensional (2D)-transthoracic echocardiography (TTE) and basic laboratory investigations like complete blood picture, fasting and postprandial blood sugar, lipid profile, blood urea and creatinine. Among patients with non-identifiable mechanism, one or more of additional investigations including 24-hours electrocardiography (Holter) monitoring, trans-esophageal echocardiography (TEE), digital subtraction angiography (DSA) of brain, and hematological investigations for systemic disorders including serum homocysteine, APLA antibodies, antithrombin-3, protein-C, protein-S, factor-V Leiden mutation and lupus anticoagulant were done. Patients of all age groups were included in this study. A total of 156 patients with cardioembolic strokes were found among 538 patients of ischemic stroke. Along with demographic details, data regarding vascular risk factors and co-morbidities was also collected. Systolic blood pressure of 130mmHg or more and/or diastolic blood pressure more than 80 was diagnosed as hypertension [5]. A diagnosis of type 2 diabetes mellitus was made in accordance with the American Diabetes Association whose criteria included: A fasting plasma glucose level of 126 mg/dL or higher (no caloric intake for at least 8 hours); An HbA1c level of 6.5% or higher; A two-hour plasma glucose level of 200 mg/dL or higher during a 75-g OGTT; A random plasma glucose of 200 mg/dL or higher in a patient with symptoms of hyperglycaemia or hyperglycaemic crisis [6].
Defining stroke subtypes
All patients with ischemic stroke were subtyped according to the trial of ORG 10172 in acute stroke treatment (TOAST) classification system into large artery atherosclerosis (LAA), cardioembolism (CE), lacunar stroke, stroke of other determined etiology, and stroke of undetermined etiology [7]. Large-artery atherosclerosis was considered in patients with significant (>50%) stenosis or occlusion as on imaging with a CT or MR angiography of major or branch cortical artery likely due to atherosclerosis. Patients with associated diabetes or hypertension with a normal computed tomography (CT)/ magnetic resonance imaging (MRI) or a relevant subcortical infarct of <1.5cm were classified as small-artery occlusion after exclusion of cardiogenic causes. Other detectable causes including vasculopathies, hematological disorders or hypercoagulable states were classified under stroke of other determined etiology. While those with all negative work up or two or more plausible etiologies were categorized as undetermined etiology.
For making a diagnosis of cardioembolic stroke, the inclusion criteria considered evidence of high-risk sources of cardiac embolism mainly from 2D echocardiography and electrocardiography. This included atrial fibrillation, rheumatic heart disease, mitral stenosis, mechanical prosthetic valve, left atrial thrombus, left ventricular thrombus, recent myocardial infarction (MI) (< 4 weeks), dilated cardiomyopathy, infective endocarditis, regional ventricular akinesia, atrial myxoma, sick sinus syndrome and patent foramen ovale [3, 7, 8]. Evidence of prior TIA or stroke in more than one vascular territory was taken as supportive evidence after exclusion of any major vessel atherosclerotic source. At least two neurologists reviewed the clinical and investigational data of an individual patient to determine the mechanism of stroke subtypes. The study was approved by the Institutional Ethics Committee of Krishna Institute of Medical Sciences.
Results
The mean age among patients with cardioembolic stroke was 61.53 years (range 28 to 65 yrs). About 69% (n=108) were men (Table 1). Significantly, a greater number of patients with cardioembolic strokes had coronary artery disease (p=0.0001) and prior history of stroke (p=0.0011) while those with non-cardioembolic strokes had greater frequency of hypertension (0.0084). Proportion of diabetes mellitus was higher among those with non-cardioembolic strokes although not statistically significant. Also, mortality rate was significantly higher in the cardioembolic stoke group (p=0.034). The etiological spectrum in the study group is shown in Table 2 and Figure 1.
Table 1: Demographic profile cardioembolic stroke.
|
Variable
|
Cardioembolic stroke
N=156 (%)
|
Non-cardioembolic stroke
N=382 (%)
|
P value
|
|
Age
|
61.532
|
61.562
|
|
|
Male
|
108(69.23)
|
253(66.23)
|
0.5446
|
|
Female
|
48(30.76)
|
129(33.76)
|
|
Hypertension
|
121(77.56)
|
333(87.17)
|
0.0084
|
|
Type 2 diabetes mellitus
|
71(45.51)
|
208(54.45)
|
0.0707
|
|
Coronary artery disease
|
56(35.89)
|
42(10.99)
|
0.0001
|
|
Previous stroke
|
52(33.33)
|
75(19.63)
|
0.0011
|
|
Mortality
|
19(12.17)
|
24(6.28)
|
0.0340
|
Table 2: Etiological spectrum of cardioembolic stroke.
|
Variable
|
n=156
|
(%)
|
|
Rheumatic heart disease
|
13(%)
|
8.33
|
|
|
Mitral valvular disease with atrial fibrillation
|
6 (46.15)
|
|
|
Mitral valvular disease without atrial fibrillation
|
2(15.38)
|
|
|
Prosthetic mitral valve with atrial fibrillation
|
1(7.69)
|
|
|
Prosthetic mitral valve without atrial fibrillation
|
1(7.69)
|
|
|
Prosthetic aortic valve without atrial fibrillation
|
1(7.69)
|
|
|
Aortic stenosis
|
1(7.69)
|
|
|
Rheumatic heart disease with infective endocarditis
|
1(7.69)
|
|
Coronary artery disease
|
56 (%)
|
35.89
|
|
|
Recent MI (<4 weeks) without clot
|
7(12.5)
|
|
|
Recent MI (<4 weeks) with clot
|
1(1.78)
|
|
|
Remote MI with regional akinesia without clot
|
25(44.64)
|
|
|
Remote MI with regional akinesia with clot
|
1(1.78)
|
|
Non-valvular atrial fibrillation
|
34
|
21.79
|
|
Left atrial cardiomyopathy
|
20
|
12.82
|
|
Post cardiac intervention
|
28(%)
|
17.94
|
|
|
CABG
|
16(57.14)
|
|
|
Others (PCI, PPI, PTCA)
|
12(42.85)
|
|
Dilated cardiomyopathy
|
5
|
3.20
|
|
Infective endocarditis
|
5
|
3.20
|
|
Sick sinus syndrome
|
1
|
0.64
|
|
Myxoma
|
1
|
0.64
|
|
Patent foramen ovale with shunt
|
1
|
0.64
|
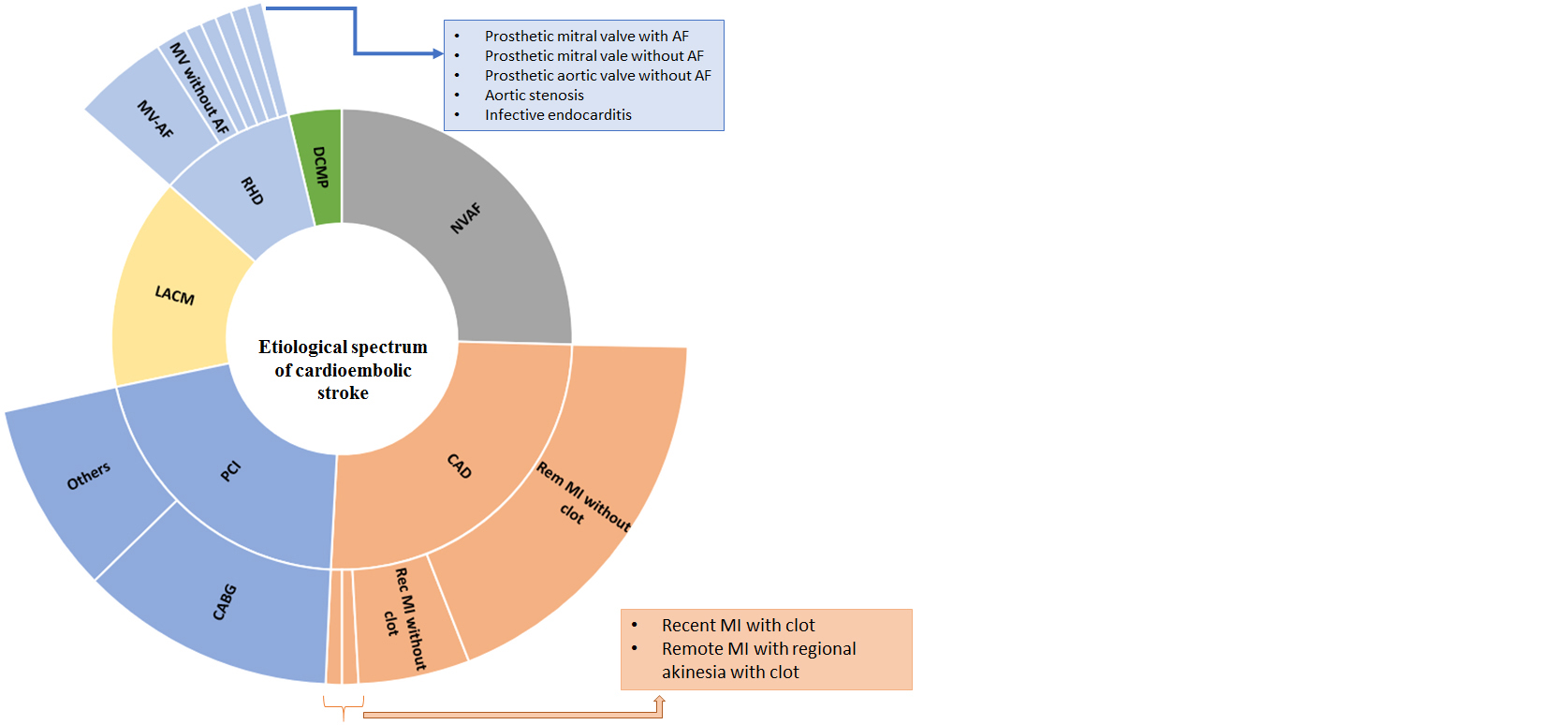
Figure 1: Etiological spectrum of cardioembolic stroke.
Discussion
In the current study, 29.9% patients with ischemic stroke were found to be cardioembolic in origin. These results are similar to western registries where 14-30 % of all cerebral infarcts were cardioembolic strokes [9-13]. Prior studies from India have shown a much lower prevalence [3, 14, 15]. The rise in detection of cardioembolic strokes in our study may be due to increased use of advanced investigations such as transesophageal echocardiography and prolonged Holter monitoring. Only one study from India showed a prevalence of 36% and since it included only the age group of 15-45 years, rheumatic heart disease was the most common cause [16].
Our study found 5 major causes of cardioembolic strokes in order of frequency; coronary artery disease (35.89%), non-valvular atrial fibrillation (21.79%), post interventional stroke (17.94%), left atrial cardiomyopathy (12.8%) and rheumatic heart disease (8.8%).
Among strokes associated with CAD, 12.5% had recent (<4 weeks) MI without left ventricular mural thrombus (LVMT) and 1.78% had recent MI with LVMT, 62% had significant regional akinesia which is a known cardioembolic source of ischemic stroke [17, 18]. From the survey of available literature, LVMT is most likely to occur by 2 weeks after an MI in 0.6–3.7% of patients, a rate which is substantially lower than the earlier reported incidence of 20–56%, possibly due to more aggressive management of acute myocardial infarction [19, 20]. Increased coagulation activity during an ACS, persisting at least up to 6 months, can potentially lead to increased thrombosis and subsequent thromboembolic events including stroke even in the absence of myocardial akinesia or visualization of thrombus in heart [21].
Cases of regional ventricular hypokinesis/ akinesia, due to remote myocardial infarction without clot (44.64%) and with clot (1.78%) formed a substantial proportion of post myocardial infarction (MI) delayed strokes. Current guidelines advocate warfarin for secondary prevention of stroke due to reduced ejection fraction < 35%, but there are no clear guidelines for primary prevention of stroke in such individuals.
Non-valvular atrial fibrillation (NVAF) was the second highest source of cardioembolic strokes in our study with the mean age of 61 years. In Western literature, the median age of AF patients is about 75 years [22]. In an Indian study, 83.63% of patients were>55years, with the majority being in the 56-65 years age [23]. This is consistent with Asian data, where the mean age of AF patients was 67.0 years±10.2 years [24].
Post procedural stroke emerged to be a frequent cardiac source of embolism out of which coronary artery bypass grafting constituted 57.14%. According to the Society of Thoracic Surgeons, Adult Cardiac Surgery Database (STS ACSD), the incidence of stroke after CABG is 1.3%, with a decrease from 1.6% to 1.2% between 2000 and 2009 [25]. The most important risk factors for stroke are age, dialysis dependency, severe chronic lung disease, emergency surgery, and atherosclerotic burden in the coronary artery and other vascular beds [26, 27]. Ischemic stroke (within 36 hours) has been reported to occur at a rate of 0.1 to 0.6 percent in patients undergoing diagnostic cardiac catheterization [28]. Meticulous attention to technical factors such as wire and catheter exchanges, high osmolar contrast agents into the carotid or vertebral arteries is necessary to minimize the to minimise the risk of complications. For patients undergoing percutaneous coronary intervention, there is some evidence that radial artery catheterization is associated with a lower risk of stroke compared with femoral artery catheterization.
Among all the cardioembolic strokes, 12.82% could be attributed to left atrial cardiomyopathy (LAC). The criteria for left atrial enlargement was taken in compliance with the American Society of Echocardiography [8]. Meanwhile, atrial cardiomyopathy was proposed as a term to describe patients with abnormal atrial substrate and function, including atrial fibrosis, atrial mechanical dysfunction, atrial electrical dysfunction, and hypercoagulable state, which can be present even without atrial fibrillation (AF). This may explain why thromboembolism can be observed even in the absence of AF.
Rheumatic heart disease accounted for 8.33% out of all the cardioembolic strokes, the prevalence of which was much less than the previous studies conducted in India [3, 16]. Other less common sources of cardioembolic strokes included dilated cardiomyopathy (3.2%), infective endocarditis (3.84%) and miscellaneous causes (1.92%), which included sick sinus syndrome, patent foramen ovale with a right to left shunt and myxoma.
Among the risk factors cardioembolic group showed significant proportion of coronary artery disease, while the traditional vascular risk factors including hypertension, diabetes mellitus and prior stroke were common among the non-cardioembolic group suggesting predisposition for small vessel disease.
Limitations
This is a retrospective data analysis based on a single center experience. However, the study does provide a wide spectrum of etiological subtypes among the category of cardioembolic stroke.
Conclusion
Cardioembolic stroke formed about one third of all ischemic strokes in this study from tertiary hospital from Hyderabad, India. The majority of cardioembolic strokes occurred as a result of complications of coronary artery disease, followed by nonvalvular atrial fibrillation, left atrial cardiomyopathy and rheumatic heart disease. It is therefore essential to look carefully for a cardiac source of embolus in all patients of ischemic stroke.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Hui C, Tadi P, Patti L. Ischemic stroke. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [Updated 2022 May 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499997/
[2] Guzmán JD. Cardioembolic stroke: Epidemiology. Neurologia. 2012; 27(Suppl 1):4–9.
[3] Manorenj S, Barla S, Jawalker S. Prevalence, risk factors and clinical profile of patients with cardioembolic stroke in South India: a five-year prospective study. Int J Community Med Public Health. 2020; 7(7):2708.
[4] Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014; 129(8):837–847.
[5] Iqbal AM, Jamal SF. Essential hypertension. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [2022 May 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539859/
[6] American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–90.
[7] Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, et al. Classification of subtypes of acute ischemic stroke. Definition for use in a multicentric clinical trial. Stroke. 1993; 24(1):35–41.
[8] Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28(1):1–39.
[9] Ferro JM. Cardioembolic stroke: An update. Lancet Neurol. 2003; 2(3):177–188.
[10] MacDougall NJ, Amarasinghe S, Muir KW. Secondary prevention of stroke. Expert Rev Cardiovasc Ther. 2009; 7(9):1103–1115.
[11] Khoo CW, Lip GY. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2009; 7(4):371–374.
[12] Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atheroscler Rep. 2006; 8(4):310–316.
[13] Di Tullio MR, Homma S. Mechanisms of cardioembolic stroke. Curr Cardiol Rep. 2002; 4(2):141–148.
[14] Manorenj S, Inturi S, Jyotsna. Trial of ORG 10172 in acute stroke treatment classification and associated risk factors of ischemic stroke: a prospective study from a tertiary care center in South India. Int J Res Med Sci. 2016; 4(11):5012–5018.
[15] Kaul S, Alladi S, Jabeen SA, Bandaru VCSSR, Ankem U, et al. Intracranial atherosclerosis is the most common stroke subtype: Ten-year data from hyderabad stroke registry (India). Ann Indian Acad Neurol. 2018; 21(3):209–213.
[16] Ganesh NS, Chinnathambi C. Cardioembolic stroke in young patients – A prospective analysis. Int J Contemp Med Res. 2019; 6(8):111–114.
[17] Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997; 336(4):251–257.
[18] Vaitkus PT, Barnathan ES. Embolic potential, prevention, and management of mural thrombus complicating anterior myocardial infarction: A meta-analysis. J Am Coll Cardiol. 1993; 22(4):1004–1009.
[19] Greaves SC, Zhi G, Lee RT, Solomon SD, MacFadyen J, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol. 1997; 80(4):442–448.
[20] Motro M, Barbash GI, Hod H, Roth A, Kaplinsky E, et al. Incidence of left ventricular thrombi formation after thrombolytic therapy with recombinant tissue plasminogen activator, heparin, and aspirin in patients with acute myocardial infarction. Am Heart J. 1991; 122(1Pt1):23–26.
[21] Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation. 1994; 90(1):61–68.
[22] Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation). Circulation. 2006; 114(7):e257–354.
[23] Jain M, Kiyawat P. Nonvalvular atrial fibrillation: A study of epidemiology, demography and clinicoetiological profile in Central India. Int J Adv Med. 2018; 5(6):1443–1449.
[24] Chien KL, Su TC, Hsu HC, Chang WT, Chen PC, et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010; 139(2):173–180.
[25] ElBardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012; 143(2):273–281.
[26] Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1–coronary artery bypass grafting surgery. Ann Thorac Surg. 2009; 88(Suppl 1):S2–S22.
[27] O’Brien SM, Feng L, He X, Xian Y, Jacobs JP, et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2-statistical methods and results. Ann Thorac Surg. 2018; 105(5):1419–1428.
[28] Korn-Lubetzki I, Farkash R, Pachino RM, Almagor Y, Tzivoni D, et al. Incidence and risk factors of cerebrovascular events following cardiac catheterization. J Am Heart Assoc. 2013; 2(6):e000413.