Full Text
Introduction
In March 2020, the World Health Organization (WHO) declared novel corona virus disease 2019 (COVID-19) as a pandemic, declaring the same as a public health emergency of great international concern [1]. With a reproduction rate of 2.5 as estimated by the WHO, the world has witnessed a rapid and widespread infection affecting patients of all age groups. Various researches have shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shares a homological sequence with SARS-CoV and MERS-CoV (family of coronaviruses), and is characterized by similar pathogenesis and manifestations [2]. The cellular entry of SARS-Cov-2 follows similar mechanism as that of SARS-CoV with ACE-2 (angiotensin-converting enzyme 2) as functional entry receptor. TMPRSS2, a cellular serine protease helps in priming of the spike protein and entry into the target cells [3]. This leads to dysregulation of RAAS (renin–angiotensin–aldosterone system) causing endothelial damage and thromboinflammation. This suggests that organs with ACE-2 receptors are at risk of COVID-19 related complications.
COVID-19 infection is more common among the elderly (>60 years) and patients with comorbidities. Patients generally become symptomatic after an incubation period of approximately 5.2 days [4]. Fever, cough, and fatigue are the most common symptoms during the initial phase of COVID-19 illness with gradual development of severe pneumonia at later stages. Other less common clinical manifestations include sore throat, headache, myalgia, arthralgia, nausea or vomiting, anosmia, nasal congestion, ageusia, diarrhea, hemoptysis, and conjunctival congestion [5, 6]. According to WHO report on COVID-19 infection, the disease has no specific disease manifestation, clinical presentation can vary from asymptomatic carriers to severe pneumonia and death [2]. Due to wide range of clinical manifestations, studies regarding multiorgan dysfunctions will help to take necessary precautions in the future.
The genetic sequence of the COVID-19 showed more than 80% identity to SARS-CoV and 50% to the Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) [7]. Research on previous SARS and MERS infections has shown multiorgan dysfunctions involving renal, hepatic, neurological, gastrointestinal and hemodynamic abnormalities [8, 9]. Since the genomic sequences and clinical manifestations between SARS, MERS, and COVID-19 are identical, the possibility of extrapulmonary manifestations and complications in COVID-19 must be evaluated for future therapeutic management of the patients.
Materials and methods
This was a retrospective observational study conducted at JSS Medical College and Hospital (JSS AHER), for a period of 3 months. The study included 145 patients who were positive for COVID-19 infection by RT-PCR test and were admitted to the hospital. The patient records between August-2020 and October-2020 were retrieved. CT was performed using a 128- slice Philips MDCT scanner (Ingenuity, Netherlands) and MR images were obtained using a 3T Philips MRI scanner (Ingenia, Netherlands). Images were evaluated using the institutional PACS database system to assess the incidence of extrapulmonary findings in COVID-19 positive patients. The clinical characteristics and incidence of extrapulmonary findings were described as frequency rates and percentages.
Results
A total of 145 COVID-19 positive patients (91 males and 54 females) were included in the study. CT and MRI were performed as per the clinical indications. Eighteen patients underwent CT brain who had presented with hemiparesis, headache, and loss of consciousness. Plain CT chest was performed for 120 patients. Six patients who had presented with acute abdomen underwent CT of the abdomen. MRI brain without contrast was done in one patient who had presented with altered sensorium and seizures. Out of 145 patients, 54 patients showed various extrapulmonary imaging findings as depicted in Table 1. Most common extrapulmonary manifestations include splenomegaly (27.7%) followed by ischemic brain infarcts (22.2%).
Table 1: Various extrapulmonary findings among the study group.
|
Patient characteristics (n=145)
|
Number (percentage)
|
|
Male
|
91 (62.7%)
|
|
Female
|
54 (37.2%)
|
|
Imaging studies (n=145)
|
|
CT Brain
|
18 (12.4%)
|
|
CT Chest
|
120 (82.7%)
|
|
CT Abdomen
|
6 (4.1%)
|
|
MRI Brain
|
1 (0.7%)
|
|
Extrapulmonary findings(n=54)
|
|
Ischemic infarct
|
12 (22.2%)
|
|
Intracranial hemorrhage
|
5 (9.2%)
|
|
Encephalitis
|
1 (1.8%)
|
|
Mediastinal lymphadenopathy
|
9 (16.6%)
|
|
Pneumomediastinum
|
4 (7.4%)
|
|
Pericardial effusion
|
2 (3.7%)
|
|
Perinephric fat stranding
|
3 (5.5%)
|
|
Splenomegaly
|
15 (27.7%)
|
|
Bowel ischemia
|
1 (1.8%)
|
|
Acute pancreatitis
|
2 (3.7%)
|
Discussion
Neurological abnormalities
Neurological manifestations in COVID-19 can involve both the central and the peripheral nervous system. Pathophysiology of neurological complications has been attributed to direct viral invasion, immunological reaction or hypoxic metabolic changes.
Common clinical manifestations are insomnia, headache, metabolic or hypoxic encephalopathy and cerebrovascular accidents, to less common features such as seizures, encephalitis, acute hemorrhagic necrotizing encephalopathy, ADEM, PRES like features, cerebral venous thrombosis and myelitis. Other common features involving the peripheral nervous system include, myalgia, anosmia, ageusia, vision loss and neuralgic pain. Rare but reported would be isolated cranial nerve palsies, Guillain-Barre syndrome, Miller-Fisher syndrome and others [10].
Radiologically, the most common occurrences were of acute and subacute infarcts (Figure 1). Other common findings included a spectrum of leukoencephalopathy, presence of micro hemorrhages, leptomeningeal contrast enhancement, cortical FLAIR signal abnormalities, and rarely is necrotizing encephalopathy [11].
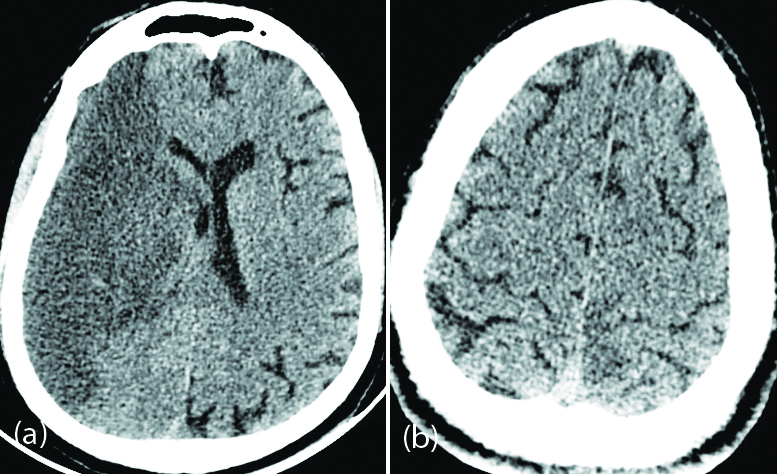
Figure 1: 60-year-old male patient with severe COVID pneumonia and left hemiparesis: (a) Axial non contrast CT of the brain shows hypodensity in the right cerebral hemisphere suggestive of right MCA territory infarction; (b) 42-year-old male patient with moderate COVID pneumonia and right hemiparesis – axial non contrast CT of the brain shows subtle hypodensity in the left parasagittal parietal cortex suggestive of ischemic changes.
Acute hemorrhagic necrotizing encephalopathy as a neurological complication in a patient with SARS-CoV-2 infection was reported in a recent study [12]. Similarly, few studies have reported the occurrence of acute ischemic stroke in COVID-19 patients [13, 14].
Cytokine storm syndrome (CSS) is a major complication in severe COVID-19 patients which can lead to acute cerebrovascular disease [15]. Further, high levels of D-dimer and thrombocytopenia in severe COVID-19 patients increases the risk of acute cerebrovascular events [16].
Viral encephalitis in COVID-19 patient scan manifest as altered mental status, abnormal behavior or speech, abnormal motor movement and focal neurological abnormalities such as flaccid paralysis, paresthesia, hemiparesis, or seizures [17]. Previously it was shown that corona virus nucleic acid was found in CSF of patients with SARS [18]. Relying on remarkable similarities between manifestations of these groups of viruses, a possibility for SARS-CoV-2 neuroinvasion should also be documented [19]. Other possible route of transmission of corona virus was found to be across the cribriform plate of the ethmoid bone and subsequently cause neuronal damage by interacting with ACE2 receptors. Additional symptoms, such as hyposmia or anosmia were found to be due to high expression of ACE-2 in nasal epithelial cells. 3D T2 FLAIR signal intensity involving olfactory bulb was greater in the patients with COVID-19 and neurologic symptoms.
Possibility of cerebrovascular endothelial rupture leading to bleeding and fatal complications was also mentioned [20]. Imaging of critically ill patients have shown microbleeds involving the corpus callosum, microbleeds in juxtacortical regions, gray-white matter interface and occasionally involving the middle cerebellar peduncles and cerebellum [21].
Acute hemorrhagic necrotizing encephalopathy on imaging presented as symmetric areas of hypoattenuation within the bilateral medial thalami on CT and hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and sub insular regions on MR imaging [12]. Symmetric areas of diffusion restriction in bilateral medial thalami, basal ganglia, medial temporal lobes, and hippocampi without hemorrhagic transformation was seen in the present study (Figure 2).
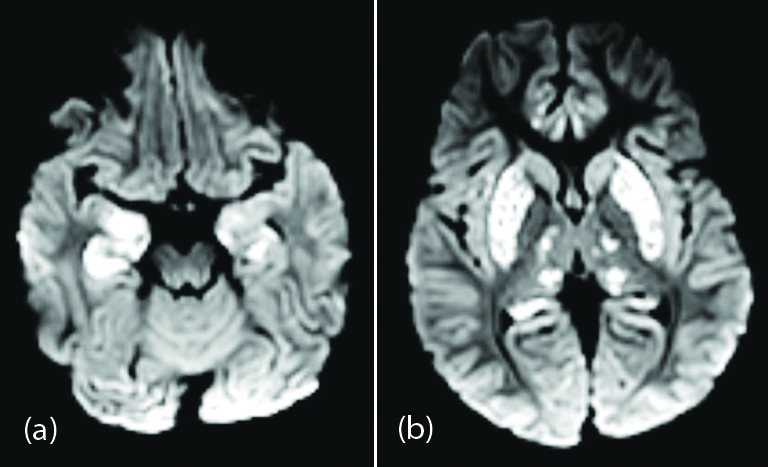
Figure 2: Two year-old male child with altered sensorium and seizures: (a),(b) axial DWI images showing symmetrical high signal areas in bilateral medial thalami, basal ganglia, medial temporal lobes, and hippocampi.
Renal involvement
Renal dysfunction in COVID-19 disease appears to be multifactorial and secondary to sepsis, comorbidities, rhabdomyolysis, treatment-related interstitial nephritis, and altered immune response [22]. Significant co-expression of ACE2 and TMPRSSs genes in podocytes and proximal convoluted tubules which makes them a potential host for SARS-CoV-2 and resulting in glomerulopathy, acute tubular necrosis, and protein leakage in the Bowman's capsule [23, 24].Various reports from China and USA have reported the occurrence of AKI in critically ill COVID-19 patients [25, 26]. Patients with both chronic kidney disease (CKD) and hypertension have an increased risk of severe COVID-19 infection [27].
CT images in patients with AKI showed reduced parenchymal density with perinephric fat stranding suggesting edema and inflammation (Figure 3). Such patients should be cautiously monitored and might require early therapeutic management to prevent further damage. Need for contrast-enhanced imaging (CT and MRI) in such patients should be avoided to prevent contrast induced nephropathy.

Figure 3: 56-year-old male patient with severe COVID pneumonia: Axial non contrast CT of the upper abdominal section shows mildly reduced renal parenchymal density with perinephric fat stranding (Arrow pointed away).
Gastrointestinal and abdominal manifestations
A significant number of patients with COVID-19 disease have presented with gastrointestinal symptoms. The overall incidence of varies from 3-70% [28]. The presence of ACE-2 receptors in the enteric epithelial tissue presumed to result in gastrointestinal symptoms such diarrhea, nausea, vomiting, and abdominal pain [29-31]. Many patients with COVID-19 disease have presented with isolated gastrointestinal manifestations without fever or respiratory symptoms [32]. There is excretion of viral particles in feces in up to 50% of COVID-19 patients and the stool samples remain positive for as much as 4 weeks [33]. This suggests a possibility of fecal-oral route of transmission.
Few studies have reported bowel abnormalities such as bowel wall thickening and findings of bowel ischemia (pneumatosis and portal venous gas) [34]. A 58-year-old male patient with COVID-19 pneumonia presented with acute abdomen and contrast CT of the abdomen showed findings of small bowel ischemia (Figure 4). Few isolated case reports have raised the suspicion that there may be an association between COVID-19 disease and acute pancreatitis (Figure 5).
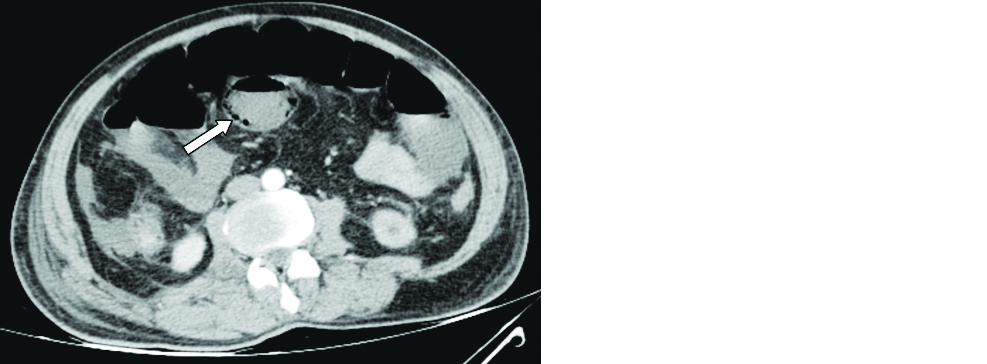
Figure 4: 58-year-old male patient with COVID pneumonia: Axial contrast CT of the abdomen revealed mildly dilated small bowel loops with intramural air (arrow) and mild interloop free fluid.
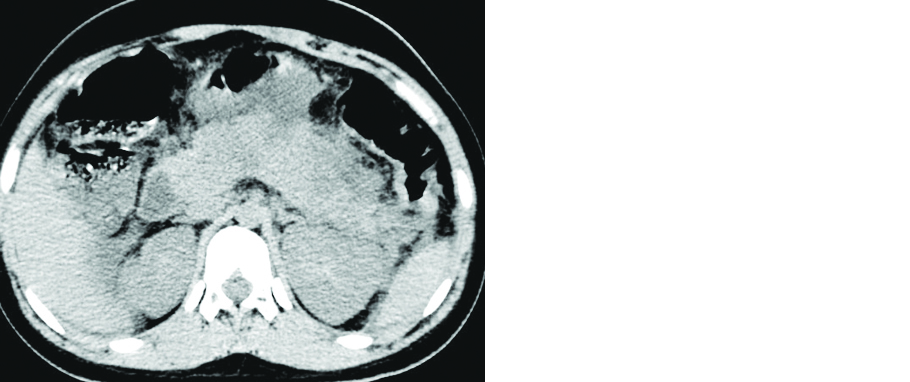
Figure 5: 21-year-old female patient with COVID pneumonia: Axial non contrast CT of the abdomen revealed bulky pancreas with hypodense areas and peripancreatic inflammatory changes (Dengue serology was negative in this patient).
Low levels of ACE-2 receptors are found in cholangiocytes resulting in direct damage to biliary ducts [35]. Further, cytokine storm syndrome (CSS) and hypoxia associated metabolic derangements also result in liver injury [36]. Various investigational drugs currently used to treat COVID-19 result in drug-induced liver injury, particularly remdesivir and tocilizumab [37]. Wide ranges of other histopathologic changes are observed which include hepatic steatosis, portal fibrosis, lymphocytic infiltrates and ductular proliferation, lobular cholestasis, and acute liver-cell necrosis [38]. This warrants long term follow up of these patients with routine liver function tests.
Mild splenomegaly has been observed as an additional finding in patients with COVID-19 disease during routine ultrasound of the abdomen and in the upper abdominal sections of the CT thorax (Figure 6). The cause appears to be non specific and similar to other viral infections [39]. Patients with severe COVID-19 infection can present with rare hyperinflammtory syndromes such as cytokine storm syndrome, secondary haemophagocytic lymphohistiocytosis (sHLH) or macrophage activation syndrome. Such patients can present with unremitting fever, cytopenias, hyperferritinaemia, and hepatosplenomegaly [40]. Few patients with COVID-19 infection presented with co-existent dengue fever. Such patients with chest symptoms should be evaluated for COVID-19 disease.

Figure 6: 42-year-old female patient with moderate severity COVID-19 pneumonia: (a) enlarged splenic shadow on scout image (arrow); (b) axial NCCT of the upper abdominal sections showing mildly enlarged spleen.
Cardiac complications
Significant higher expression of the ACE-2 in cardiac tissues such as cardiac myocytes, fibroblasts, endothelial cells, and smooth-muscle cells appears to be the likely cause of cardiovascular complications [41]. Most common manifestations include myocarditis, acute coronary syndromes (ACS), cardiomyopathy, acute cor pulmonale (ACP), arrhythmias, and cardiogenic shock [42]. Myocardial injury was indicated by increased levels of cardiac bio-markers such as cardiac troponin I (cTnI), creatine kinase (CK), α-hydroxybutyrate dehydrogenase (HBDB), and lactate dehydrogenase (LDH) [43]. Apart from myocardial ischemia, cardiac arrhythmias have been observed in COVID-19 patients without a previous history of cardiac diseases. These patients might remain asymptomatic or present with palpitations [42]. Most patients with arrhythmias show some type of ECG changes including sinus tachycardia, sinus bradycardia, QTc prolongation (often drug induced), torsades de pointes and paroxysmal atrial fibrillation [44]. Routine electrocardiogram monitoring is needed in all critically ill patients and especially with a previous history of ischemic heart disease or cardiovascular risk factors. The mortality rate is found to be higher in patients with CVS comorbidities [45]. Cardiomegaly and pericardial effusion are the most common incidental cardiac imaging findings in patients with COVID-19 disease (Figure 7).
Long term follow up of these infected patients (cardiac biomarkers and echocardiogram) are needed to assess the changes in cardiac functional parameters.
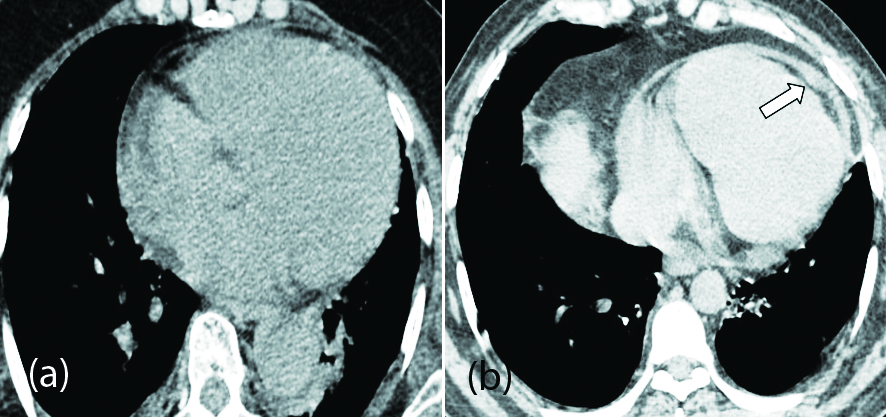
Figure 7: (a) axial CT chest image of 70-year-old female patient with mild COVID-19 pneumonia showing cardiomegaly; (b) axial CT chest image of 54-year-old female patient with minimal pericardial effusion (arrow).
Mediastinal complications
Mediastinal lymphadenopathy is not a typical imaging feature in COVID-19 disease as described in previous studies [5]. However, enlarged mediastinal nodes was a common finding in patients with more severe infection [46]. Lymphadenopathy could be due to secondary infections or reactive phenomenon to viral disease (Figure 8a).
Spontaneous pneumomediastinum is another rare mediastinal complication observed in COVID-19 patients. Various case reports have been described regarding development of spontaneous pneumothorax and pneumomediastinum in COVID-19 patients without assisted ventilation [47, 48]. The rupture of alveoli secondary to diffuse alveolar injury in patients with severe COVID-19 pneumonia could be the cause for spontaneous pneumomediastinum (Figure 8b). An increase in the intra-alveolar pressure results in the alveolar rupture and migration of free air into the mediastinum which is referred as Macklin effect [49].

Figure 8: (a) 55-year-old male patient with mild COVID-19 pneumonia and enlarged mediastinal lymph nodes (arrows); (b) 36-year-old male patient with severe COVID-19 pneumonia complicated with pneumomediastinum (arrow).
Hematological manifestations
COVID-19 angiopathy or vasculopathy appears to be an emerging hematological complication in patients recovering from COVID-19 pneumonia. Elevated levels of D-dimer and fibrinogen are markers of COVID-19-associated vasculopathy [50]. Severe thromboinflammation results in hypercoagulability, endothelial damage, complement activation and other mechanisms which increase the risk of venous thromboembolism [51]. CT pulmonary angiography and ultrasound imaging can be used in patients with elevated levels of D-dimer and high suspicion of pulmonary thromboembolism (PTE) and deep vein thrombosis (DVT). The pulmonary involvement in COVID-19 pneumonia has been attributed to microvascular thrombosis [52]. Lymphopenia, neutrophilia and thrombocytopenia are other markers of COVID-19 infections [53].
Ocular and dermatological manifestations
Ocular involvement in COVID-19 patients is uncommon and has low prevalence [54]. The presentation is similar to follicular conjunctivitis with increased secretions, chemosis, ocular irritation, and foreign body sensation. RTPCR test with conjunctival swabs of patients with ocular symptoms have shown positive results suggestive of viral replication in the conjunctiva [55]. A rare case report of central retinal artery occlusion has been described which needs to be substantiated with further studies [56].
Dermatological manifestations are of less significance in COVID-19 patients and do not correlate with disease severity. Major cutaneous manifestations include maculopapular rash, papulovesicular rash, urticaria, painful acral red-purple papules, livedo reticularis lesions, and petechiae [57].
Others
Recent studies have shown high ACE2 receptor expression in the testicular cells might result in reproductive disorders through abnormal activation of ACE2 pathway [58]. Scrotal ultrasound in COVID-19 patients with testicular pain might be useful in the detection of the viral orchitis which is the most common manifestation.
Conclusion with future implications
There is a substantial increase in the number of COVID-19 cases and associated extrapulmonary manifestations that need to be familiarized. The present study illustrates the incidence of neurological, renal, gastro-abdominal, hepato-biliary, cardiac, and mediastinal findings in COVID-19 patients. Further analysis of data from a larger patient cohort is necessary before the pandemic reaches a second peak. At present more attention is paid to the pulmonary manifestation of COVID-19 pneumonia. It is important for both clinicians and radiologists to understand and anticipate extrapulmonary complications in patients with COVID-19 disease to improve the clinical outcome.
Conflicts of interests
Authors declare no conflicts of interest.
References
[1] WHO COVID-19 is a pandemic. 2020.
[2] WHO. Report of the WHO-China joint mission on corona virus disease 2019 (COVID-19) 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
[3] Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271–280.
[4] Li Q, Guan X, Wu P, Wang X, Zhou L, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020; 382(13):1199–1207.
[5] Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel corona virus in Wuhan, China. Lancet. 2020; 395(10223):497–506.
[6] Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020; 75(7):1730–1741.
[7] Lu R, Zhao X, Li J, Niu P, Yang B, et al. Genomic characterisation and epidemiology of 2019 novel corona virus: implications for virus origins and receptor binding. Lancet. 2020; 395(10224):565–574.
[8] Gu J, Gong E, Zhang B, Zheng J, Gao Z, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005; 202(3):415–424.
[9] Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, et al. Middle East respiratory syndrome. N Engl J Med. 2017; 376(6):584–594.
[10] Garg RK. Spectrum of neurological manifestations in COVID-19: A Review. Neurol India 2020; 68(3):560–572.
[11] Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020; 382(23):2268–2270.
[12] Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, et al. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020; 296(2):E119–E120.
[13] Mao L, Jin H, Wang M, Hu Y, Chen S, et al. Neurologic Manifestations of Hospitalized Patients With Corona virus Disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6):683–690.
[14] Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191:9–14.
[15] Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. The Lancet. 2020; 395(10229):1033–1034.
[16] Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel corona virus pneumonia (COVID19) implicate special control measures. J Med Virol. 2020; 92(6):568–576.
[17] Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, et al. Human corona viruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system?. Viruses. 2019; 12(1):14.
[18] Marc D, Dominique JF, Elodie B. Human corona virus: respiratory pathogens revisited as infectious neuroinvasive, neurotropic, and neurovirulent agents. CRC Press; 2013:93–122.
[19] Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008; 82(15):7264–7275.
[20] Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the cns: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020; 11(7):995–998.
[21] Cannac O, Martinez-Almoyna L, Hraiech S. Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology. 2020 Sep 15; 95(11):498–499.
[22] Gulati A, Pomeranz C, Qamar Z, Thomas S, Frisch D.A comprehensive review of manifestations of novel corona viruses in the context of deadly COVID-19 global pandemic. Am J Med Sci. 2020; 360:5–34.
[23] Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nat 2003; 426(6965):450–454.
[24] Su H, Yang M, Wan C, Yi LX, Tang F, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020; 98(1):219–227.
[25] Yang X, Yu Y, Xu J, Shu H, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8(5):475–481.
[26] Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020; 395(10239):1763–1770.
[27] Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020(6):1–2.
[28] Tian Y, Rong L, Nian W, He Y. Gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment PharmacolTher 2020; 51(9):843–851.
[29] Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020; 158(6):1518–1519.
[30] Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506.
[31] Wu F, Zhao S, Yu B, Chen YM, Wang W, et al. A new coronavirus associated with human respiratory disease in China. Nat 2020; 579(7798):265–269.
[32] Aroniadis OC, DiMaio CJ, Dixon RE, Elmunzer BJ, Kolb JM, et al. Current knowledge and research priorities in the digestive manifestations of COVID-19. Clin Gastroenterol Hepatol. 2020; 18(8):1682–1684.
[33] Wu Y, Guo C, Tang L, Hong Z, Zhou J, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020; 5(5):434–435.
[34] Bhayana R, Som A, Li MD, Carey DE, Anderson MA, et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020; 297(1):E207–E215.
[35] Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020; 5(5):428–430.
[36] Li H, Liu L, Zhang D, Xu J, Dai H, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020; 395(10235):1517–1520.
[37] Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020; 5(6):529–530.
[38] Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020; 173(5):350–361.
[39] Tabatabaei SMH, Talari H, Moghaddas F, Rajebi H. Computed tomographic features and short-term prognosis of coronavirus disease 2019 (COVID-19) pneumonia: a single-center study from Kashan, Iran. Radiology: Cardiothoracic Imaging. 2020; 2(2):e200130.
[40] Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229):1033–1034.
[41] Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008; 295(6):H2373–H2379.
[42] Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020; 75(18):2352–2371.
[43] Zhou B, She J, Wang Y, Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J Infect. 2020; 81(1):147–178.
[44] Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061–1069.
[45] Chen N, Zhou M, Dong X, Qu J, Gong F, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel corona virus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223):507–513.
[46] Valette X, du Cheyron D, Goursaud S. Mediastinal lymphadenopathy in patients with severe COVID-19. Lancet Infect Dis. 2020; S1473-3099(20):30310–8.
[47] Sun R, Liu H, Wang X. Mediastinal Emphysema, Giant Bulla, and Pneumothorax Developed during the Course of COVID-19 Pneumonia. Korean J Radiol. 2020; 21(5):541–544. [48] Wang J, Su X, Zhang T, Zheng C. Spontaneous Pneumomediastinum: A Probable Unusual Complication of Coronavirus Disease 2019 (COVID-19) Pneumonia. Korean J Radiol. 2020; 21(5):627–628.
[49] Murayama S, Gibo S. Spontaneous pneumomediastinum and Macklin effect: Overview and appearance on computed tomography. World J Radiol. 2014; 6(11):850–854.
[50] Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020; 135(23):2033–2040. [51] Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020; 18(7):1559–1561.
[52] Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translat Res. 2020; 220:1–13.
[53] Zhou F, Yu T, Du R, Fan G, Liu Y, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062.
[54] Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708–1720.
[55] Wu P, Duan F, Luo C, Liu Q, Qu X, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020; 138(5):575–578.
[56] Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020; 21:e00867.
[57] Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020; 183(1):71–77.
[58] Shen Q, Xiao X, Aierken A, Yue W, Wu X, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020; 24(16):9472–9477.