Full Text
Introduction
As compared with the rest of the skeleton, benign neoplasms of the axial skeleton particularly vertebrae are very rare. In the literature, several articles have been published under individual neoplasms, but as a group of benign neoplasms the publications are scarce, particularly with regards to imaging. This article is an attempt to describe the imaging findings of benign neoplasms of the vertebrae and to arrive at a correct diagnosis prior to therapeutic management. Benign neoplasms of the vertebrae include hemangioma, giant cell tumor, osteoid osteoma, osteoblastoma and osteochondroma (Table 1). However, tumors like conditions are many which include fibrous dysplasia, Paget’s, eosinophilic granuloma and aneurysmal bone cyst (Table 2). It is quite essential to identify tumor like lesions as not infrequently, the patients are subjected to unnecessary costly investigations, biopsies and even surgery.
Table 1: Benign neoplasms of spine.
• Osteoid osteoma
• Osteoblastoma
• Osteochondroma
• Chondromyxoid fibroma
• Chondroblastoma
• Hemangioma
• Giant cell tumor
Table 2: Tumoral lesions of the spine.
• Enostosis - Bone island
• Aneurysmal bone cyst
• Eosinophilic granuloma
• Fibrous dysplasia
• Paget’s
Imaging methods: Conventional; MDCT; MRI; Angiography; Pet CT
Review of the literature
Greenspan has extensively described the radiological and pathological features of bone forming lesions like bone island, osteoid osteoma, osteoblastoma etc. [1]. He also has described radiological features of tumor like lesions [2]. Assoun and Davies et al have described the MRI features of osteoid osteoma [3, 4]. Harish et al. have described imaging features of spinal osteoid osteoma [5]. Obenberger et al. have described the imaging features of osteoblastoma [6]. Lucas et al. have described the clinicopathological features of osteoblastoma [7]. Shaikh et al have described CT and MR features of spinal osteoblastoma [8]. Radiologicpathologic correlation of primary tumours of spine have been described by Murphey et al., [9]. Fluid-fluid level: a nonspecific finding in tumors of bone and soft tissue have been described by Tsai et al., [10]. Imaging of osteochondroma, its variants and complications with radiologicpathologic correlation have been detailed by Murphey et al., [11]. Vertebral tumors and pseudotumors have been discussed by Laredo et al., [12]. Vertebral chondroblastomas have been described by Ilaslan and Vialle et al., [13, 14]. Vertebral hemangiomata have been elaborately described by Baudre et al., [15-17]. Giant cell tumours have been described by Kwon et al., [18]. Aneurysmal bone cysts have been discussed by Papagelopoulos et al., [19, 20]. Langerhans’ cell histiocytosis of the spine has been described by Yeom et al., [21]. Fibrous dysplasia localized to spine: a diagnostic dilemma has been published by Gogia et al., [22]. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation have been described by Smith et al., [23]. However, no single paper has been published regarding benign tumors of vertebrae and discussed the differential diagnosis. T
his paper consists of the spectrum of benign tumors of the vertebral column and the imaging findings, keeping in view of the previous literature.
Osteoid osteoma (OO): 10% occur in the vertebrae; Painful scoliosis, radicular pain; Size extend to 2-10mm in diameter; Incidence 3-20 yrs; Male to female ration 2:1; Most of them are located in posterior elements; Giant osteoid osteoma in the spine is rare.
Imaging findings: Round lucent lesion with a nidus (Figure 1a,b,c); Central calcifications are common; CT is the best imaging methods (Figure 1d,e,f,g); Scintigraphy is sensitive but not specific; MRI is rarely necessary which shows low signal in T1 and high in T2. Calcium low signal in all images.
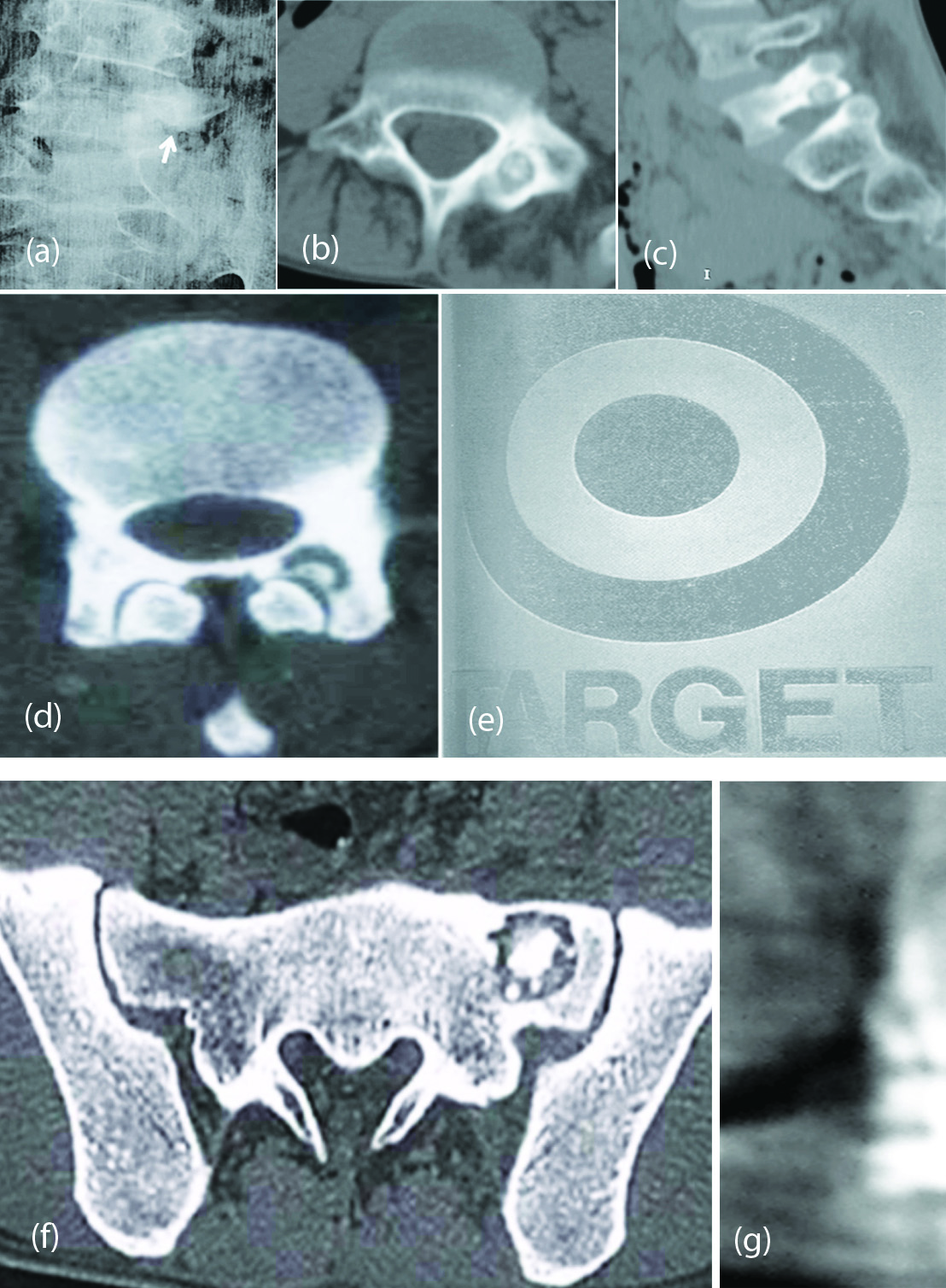
Figure 1: (a, b, c) Osteoid osteoma L5 posterior element. (d, e) CT - Target sign – Osteoid osteoma of lamina. (f) CT- Osteoid osteoma. (g) Osteoid osteoma of T11.
In the differential diagnosis of a dense / ivory pedicle, metastasis (Figure 2a), compensatory hypertrophy where contra lateral spondylolysis (Figure 2b) is present and where the contra lateral pedicle is absent (Figure 2c).
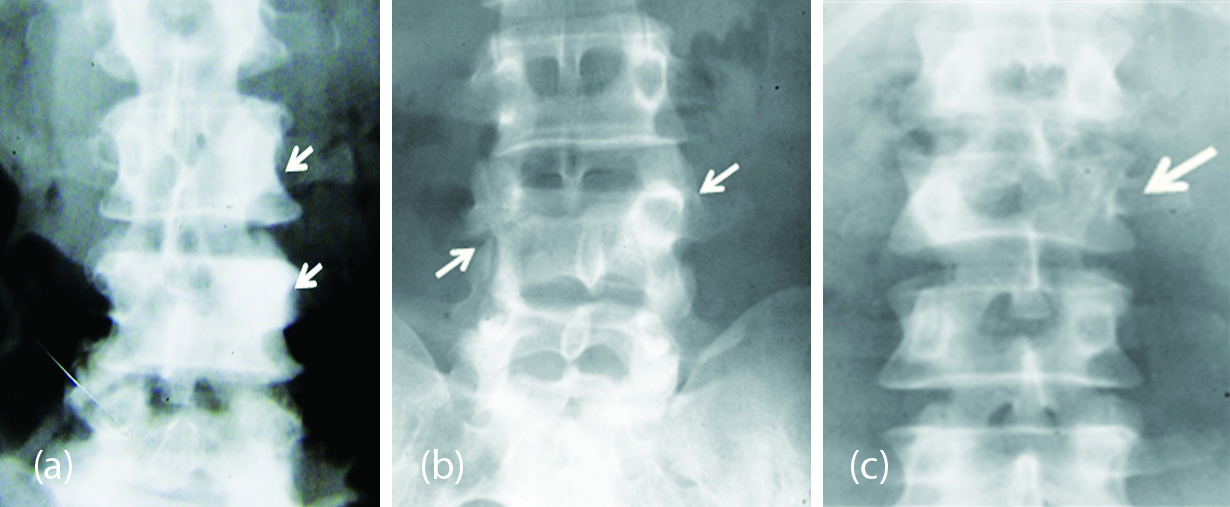
Figure 2: (a) Dense pedicles on the left due to metastasis. (b) Dense left pedicle of L4 with spondylolysis on the right side. (c) Dense right pedicle of L2 with absence of left pedicle.
Osteoblastoma: Nidus larger than 2 cm; 90% occur in the 2nd and 3rd decades; Occurs in the posterior elements of the spine; May be associated with aneurysmal bone cyst.
Imaging findings
Four types morphologically: 1. Osteoid osteoma type (Figure 3a); 2. Aneurysmal bone cyst type (Figure 3b); 3. Osteosarcoma type (Figure 3cd); 4. Fibrous dysplasia type (Figure 3e). CT is ideal imaging method (Figure 3f); scintigraphy and MRI are nonspecific.

Figure 3: (a) Osteoblastoma L4 at right side. (b) Osteoblastoma C2 spinous process. (c) Osteoblastoma (Osteosarcoma type) of L3. (d) Osteoblastoma C3 spinous process. (e) Osteoblastoma (Fibrous dysplasia type) – C2. (f) CT - Osteoblastoma of lamina of thoracic vertebra.
Cartilaginous tumors
Cartilaginous tumors are very rare in the vertebrae: Osteochondroma may be seen either isolated or part of multiple heriditary osteochondromata; Generally arises from posterior elements; Usually small in size; Produce neurological symptoms when they grow into the spinal canal.
Imaging methods & findings
Plain film findings are characteristic resembling a cauliflower like growth. The cortex extends from the host bone along the osteochodroma. Cartilaginous calcifications may be seen at the cap (Figure 4a,b,c,d). CT is the best examination to show calcifications (Figure 4e,f,g,h); MRI is rarely necessary (Figure 4i). Malignant transformation is rare.

Figure 4: (a, b) Osteochondroma of L4. (c) Osteochondromata of cervical spine. (d) Osteochondroma of the spinous process of cervical spine. (e) CT - Osteochondroma of the transverse process. (f) Osteochondroma arising from the left transverse process of L1. (g, h) Osteochondroma of L4 spinous process. (i) MRI – Osteochondroma of cervical spine.
Chondromyxoid fibroma and chodnroblastoma of the vertebrae have been described very rarely.
Vertebral hemangioma
Vertebral hemangioma is also known as hamartoma. One of the commonest benign tumours of the vertebral column. The vast majority of these are asymptomatic. Infrequently, these can turn symptomatic and cause neurological deficit (cord compression) through any of four reported mechanisms: 1. Epidural extension; 2. Expansion of the involved vertebra(e) causing spinal canal stenosis; 3. Spontaneous epidural haemorrhage; 4. Pathological burst fracture. Vertebral hemangioma can be single or multiple, more common in women, and may be part of cystic angiomatosis
Imaging findings
Plain film – AP and lateral views: (a) A well demarked, lucent lesion with coarse trabeculations, (b) Cortical expansion may be seen but no cortical invasion, (c) May involve the whole body and extend to the posterior elements, (d) Can be small and isolated, (e) Spoke wheel pattern when small, (f) Jail bar appearance with vertical striations due to resorbed primary trabeculae (Figure 5a) and reinforced secondary trabeculae, (g) Sclerosing hemangioma may show marked sclerosis with lucent and coarse trabeculations (Figure 5b).
CT is ideal investigation to show the expansion and residual trabeculae with Polka dot appearance(Figure 5c,d,e,f,g,h), Angiography is rarely necessary. However, it shows hypervascularity (Figure 5i), scintigraphy is not specific, MRI shows low signal in T1 and high signal in T2 (Figure 5j,k,l).
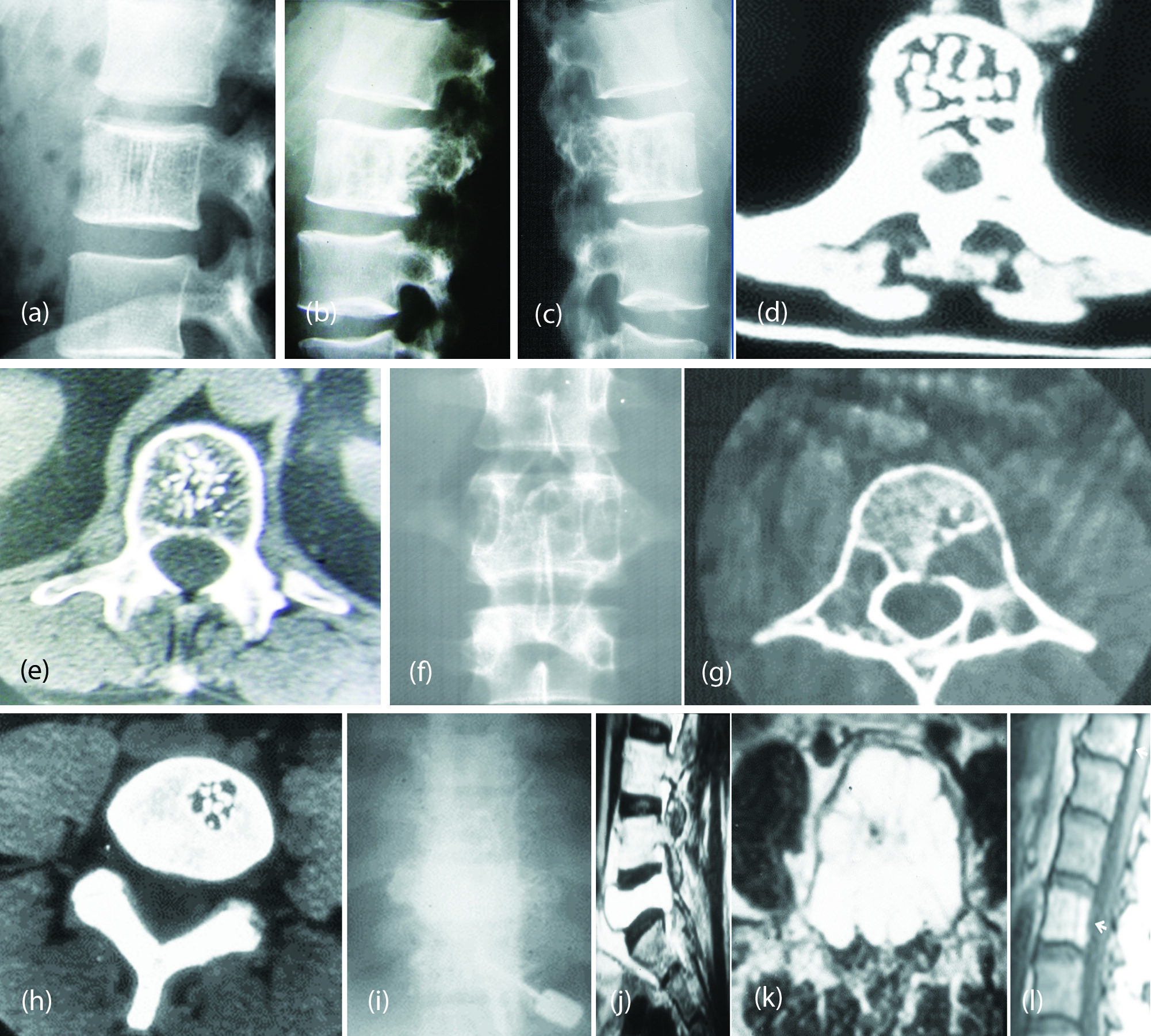
Figure 5: (a) Hemangioma of L2 with “Jail Bar” sign. (b) Sclerosing hemangioma of L2. (c, d) Hemangioma of L3 - CT Polka dot. (e) CT – Hemangioma “Polca Dot” appearance. (f, g) Cavernous hemangioma of L2 – CT. (h) CT small hemangioma. (i) Hemangioma of vertebra – angiogram. (j, k) MR Hemangioma of L5 with hyperintensity signals in T2. (l) MRI Multiple hemangiomata of lumbar spine showing bright signals.
Giant cell tumor (GCT)
GCT of the spine is very rare except in the sacrum. The radiological principles applied in long bone giant cell tumors may be applied in the spine.
Imaging findings
Plain film – (a) Well defined large lytic lesion without any mineralized matrix, (b) The transiltional zone is narrow, (c) The average age is 33 years, (d) Primary malignant GCT is very rare in the spine.
CT defines the margins much better than seen in plain films (Figure 6a,b,c), CT and MRI are helpful in showing the aggressive nature of the lesion. Fluid-fluid level may or may not be present except when ABC is associated with GCT (Figure 6def), Scintigraphy is non-specific.

Figure 6: (a) CT – Giant cell tumor of sacrum. (b, c) CT - Giant cell tumor of L5. (d, e, f) MRI - Giant Cell Tumor of the sacrum, no fluid-fluid levels.
Tumoral lesions of the spine (Benign)
Aneurysmal bone cyst (ABC)
Primary & Secondary: Enostosis – Bone island; Primary ABC; 80% occur between the ages of 5 and 30; Secondary ABC occur according the age of the primary lesion; Posterior elements are involved in the vertebrae; Invariably extend to the body of the vertebra. Enostosis - Bone Island Radiological findings: Can occur in the vertebrae; Asymptomatic; Sclerotic lesion (Figure 7a); Margins may be spiculated (Figure 7b); Small size varying from 2-8mm (Figure 7c); Giant bone islands also have been reported.

Figure 7: (a, b) CT – Bone island – Thoracic vertebra. (c) CT – Bone island.
Primary aneurysmal bone cyst (ABC)
It is encountered in first three decades of life. It occurs equally in both males and females. Over half of them occur in long bones mainly in metaphysic. Vertebral bodies and posterior elements are a common site.
Imaging findings
Plain film findings: (a) Expansile lytic lesion eccentrically located (Figure 8a,b,c,d), (b) Narrow zone of transition, (c) No periosteal reaction, unless there is a microfracture, (d) Residual trabeculae maybe noted in the matrix, (e) May extend from the vertebral body to posterior elements. CT and MRI show multiple fluid-fluid levels (Figure 8efg); Scintigraphy is non specific; Angiography may not be helpful.

Figure 8: (a) Aneurysmal bone cyst C2 spinous process. (b) Aneurysmal bone cyst of L4 – Lytic expanding lesion with sclerotic rim. (c, d) Aneurysmal bone cyst L3. (e) Aneurysmal bone cyst – Sacrum. (f, g) Aneurysmal Bone Cyst of spinous process of L1. Note the fluid-fluid levels.
Secondary aneurysmal bone cyst (ABC)
It has been observed in benign lesions such as giant cell tumor, chondroblastoma, fibrous dysplasia, nonossifying fibroma and in malignant lesions such as osteosarcoma particularly telangiectatic osteosarcoma. The number of fluid-fluid levels often determines whether it is primary or secondary.
Eosinophilic granuloma (EG - Langerhan’s histiocytosis X)
Langerhan’s Histiocytosis X includes Litter Siwe’s disorder in infants, Hand Schuller disease in young adults and eosinophilc granuloma at any age. EG often involves the vertebral body than its appendages.
Plain film findings: Lytic lesion involving the vertebral body (Figure 9a,b); Vertebra plana; CT findings (Figure 9c); Confirm the plain film findings.
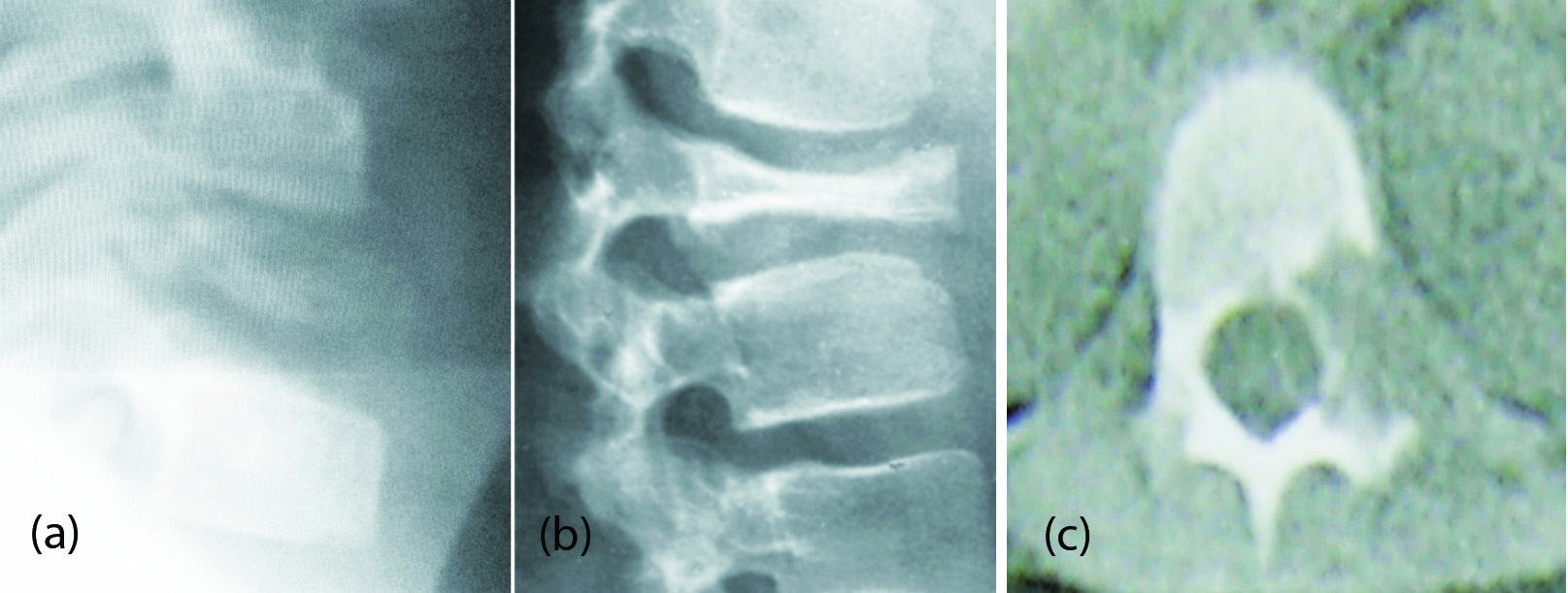
Figure 9: (a, b) Vertebral plana – Eosinophilic granuloma. (c) CT - Eosinophilic granuloma.
Fibrous dysplasia (FD)
FD is a developmental disorder of the bone with fibrous replacement of the bone in a bizarre fashion. It manifests as 1. Monostotic 2. Polyostotic and 3. Polyostotic with cutaneous and endocrinal changes particularly in girls and is called Albright Syndrome. FD of the vertebrae in monostotic form is quite rare. It is occasionally encountered in polyostotic fibrous dysplasia. Thoracic and lumbar vertebrae as well as sacrum are affected mostly.
Imaging findings
Plain film findings: The normal architecture of the vertebral body is disturbed by fibrous tissue and new bone formation. This may extend to vertebral appendages. The matrix is often shows ground glass appearance. The entire morphology is disturbed with mixed lysis and sclerosis (Figure 10a,b,c).
CT findings: CT and MRI do not manifest any additional findings than are described on plain films (Figure 10d).
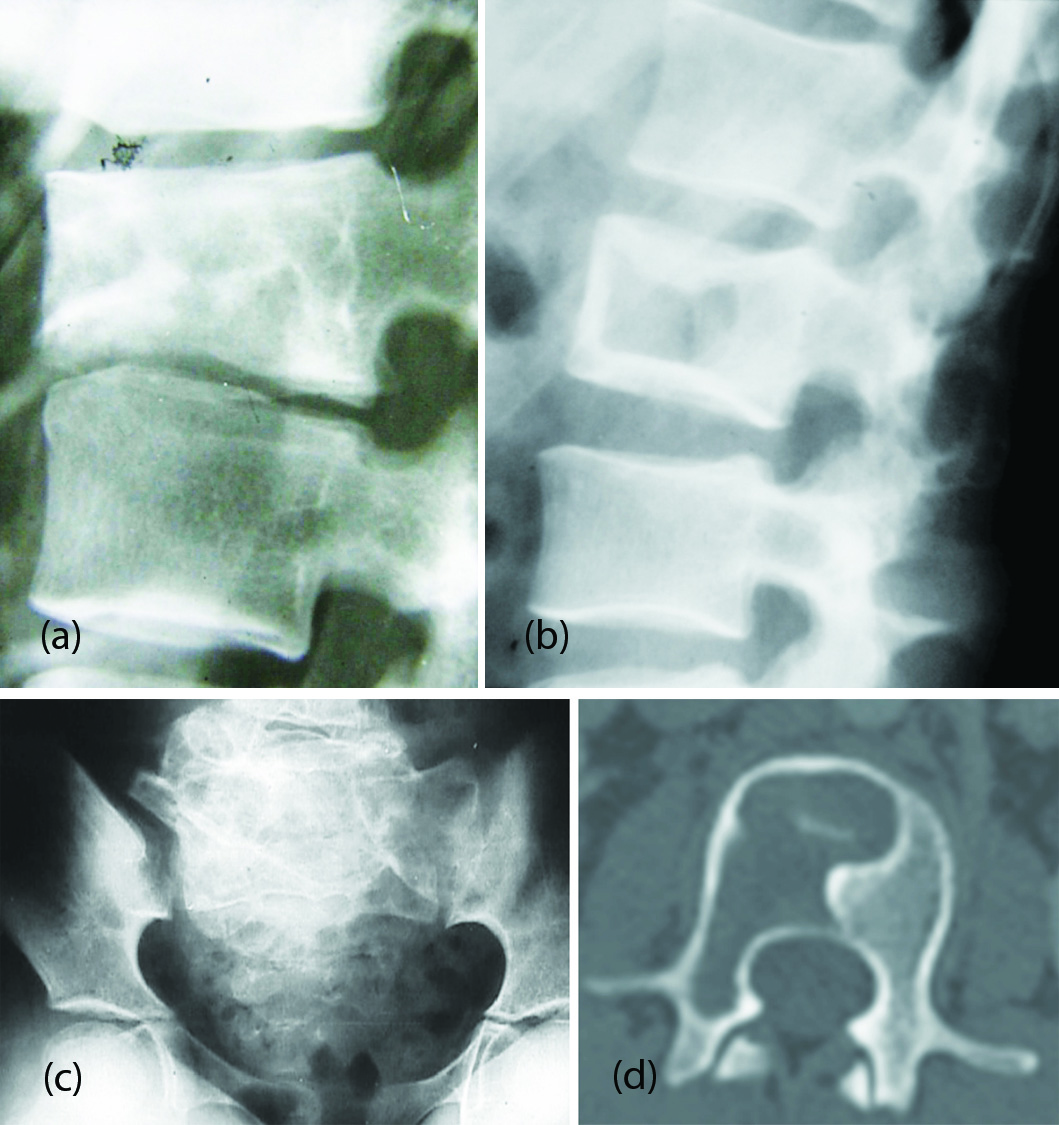
Figure 10: (a) Fibrous dysplasia of L2 with compression and ground glass appearance. (b) Fibrous dysplasia of L1. Picture frame appearance simulating Paget’s. (c) Fibrous dysplasia of the sacrum- Deformity with bizare trabeculations. (d) CT – L2 Fibrous dysplasia showing lytic area with sclerotic border.
Paget’s disease
Paget’s disease is very rare but involvement of the vertebra is not uncommon. This can be monostotic but often is polyostotic. Complications may include fractures, compression on the spinal cord and rarely malignant transformation.
Imaging findings
Plain film may show lysis with compression fracture. In the mixed phase the findings include both lysis and sclerosis (Figure 11a). It may involve more than one vertebra (Figure 11b). In the sclerotic phase, the vertebral body is dense and is called ivory vertebra (Figure 11c).
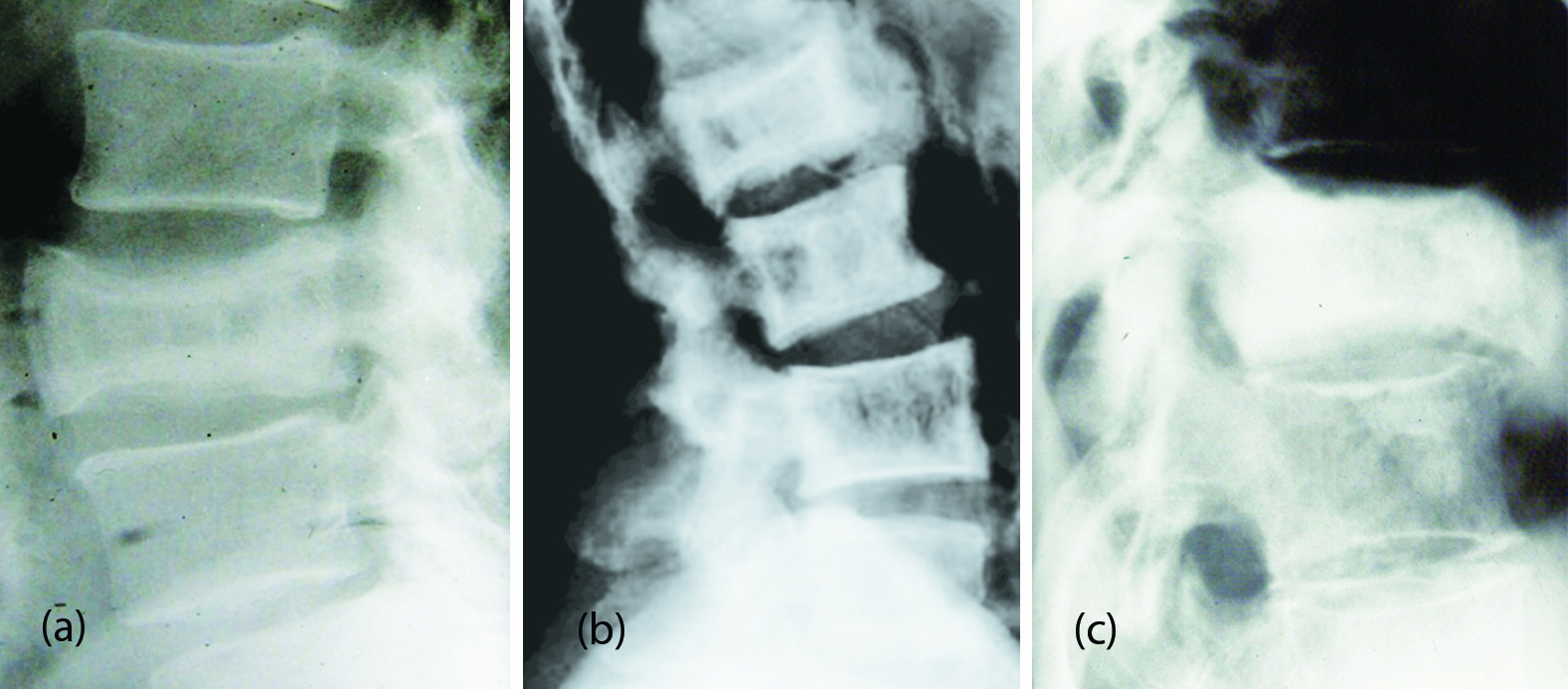
Figure 11: (a) Paget’s of L3 – Sclerosis with enlargement of body. (b) Paget’s disease involving multiple lumbar vertebrae. (c) Paget’s disease with complete sclerosis (Ivory Vertebra).
Conclusion
Benign neoplasms of the vertebrae are quite rare. However, the conventional radiological findings are quite characteristic of each entity. Advanced imaging is rarely necessary for confirming the diagnosis. Of all the neoplasms, hemangioma of the vertebra is most common benign neoplasm. Tumoral lesions of the vertebrae such as bone island, aneurysmal bone cyst, eosinophilic granuloma etc., are discussed with differentiating points from imaging point of view.
Acknowledgements
Nizam’s Institute of Medical Sciences (NIMS), Hyderabad, and Kakarla Subbarao Radiological & Imaging Educational Sciences Trust (KREST), Hyderabad, India.
Conflicts of interest
Author declares no conflicts of interest.
References
[1] Greenspan A. Benign bone-forming lesions: Osteoma, osteoid osteoma, and osteoblastoma-clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993; 22(7):485–500.
[2] Greenspan A. Radiologic evaluation of tumors and tumor-like lesions. In: Orthopedic imaging: A practical approach. 4th edition Philadelphia. Pa: Lippincott Williams & Wilkins. 2004; 529–570.
[3] Assoun J, Richardi G, Railhac JJ, Baunin C, Fajadet P, et al. Osteoid osteoma: MR imaging versus CT. Radiology. 1994; 191(1):217–223.
[4] Davies M, Cassar-Pullicino VN, Davies AM, Mc-Call IW, Tyrrell PN. The diagnostic accuracy of MR imaging in osteoid osteoma.Skeletal Radiol. 2002; 31(10):559–569.
[5] Harish S, Saifuddin A. Imaging features of spinal osteoid osteoma with emphasis on MRI findings. Eur Radiol. 2005; 15(12):2396–2403.
[6] Obenberger J, Seidl Z, Plas J. Osteoblastoma in lumbar vertebral body. Neuroradiology. 1999; 41(4):279–282.
[7] Lucas DR, Unni KK, McLeod RA, O’Connor MI, Sim FH. Osteoblastoma: Clinicopathologic study of 306 cases. Hum Pathol. 1994; 25(2):117–134.
[8] Shaikh MI, Saifuddin A, Pringle J, Natali C, Sherazi Z. Spinal osteoblastoma: CT and MR imaging with pathological correlation. Skeletal Radiol. 1999; 28(1):33–40.
[9] Murphey MD, Andrews CL, Flemming DJ, Temple HT, Smith WS, et al. Primary tumors of the spine: Radiologicpathologic correlation. Radiographics. 1996; 16(5):1131–1158.
[10] Tsai JC, Dalinka MK, Fallon MD, Zlatkin MB, Kressel HY. Fluid-fluid level: A nonspecific finding in tumors of bone and soft tissue. Radiology. 1990; 175(3):779–782.
[11] MurpheyMD, Choi JJ, Kransdorf MJ, Flemming DJ, Gannon FH. Imaging of osteochondroma: variants and complications with radiologicpathologic correlation. RadioGraphics. 2000; 20(5):1407–1434.
[12] Laredo JD, el Quessar A, Bossard P, Vuillemin-Bodaghi V. Vertebral tumors and pseudotumors. Radiol Clin North Am. 2001; 39(1):137–163.
[13] Ilaslan H, Sundaram M, Unni KK. Vertebral chondroblastoma. Skeletal Radiol. 2003; 32(2):66–71.
[14] Vialle R, Feydy A, Rillardon L, Tohme-Noun C, Anract P, et al. Chondroblastoma of the lumbar spine: Report of two cases and review of the literature. J Neurosurg Spine. 2005; 2(5):596–600.
[15] Baudrez V, Galant C, Vande Berg BC. Benign vertebral hemangioma: MR-histological correlation. Skeletal Radiol. 2001; 30(8):442–446.
[16] CrossJJ, Antoun NM, Laing RJ, Xuereb J. Imaging of compressive vertebral haemangiomas. Eur Radiol. 2000; 10(6):997–1002.
[17] PastushynAI, Slin’ko EI, Mirzoyeva GM. Vertebral hemangiomas: Diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surg Neurol. 1998; 50(6):535–547.
[18] Kwon JW, Chung HW, Cho EY, Hong SH, Choi SH, et al. MRI findings of giant cell tumors of the spine. AJR Am J Roentgenol. 2007; 189(1):246–250.
[19] Papagelopoulos PJ, Currier BL, Shaughnessy WJ, Sim FH, Ebsersold MJ, et al. Aneurysmal bone cyst of the spine: Management and outcome. Spine. 1998; 23(5):621–628.
[20] Suzuki M, Satoh T, Nishida J, Kato S, Toba T, et al. Solid variant of aneurysmal bone cyst of the cervical spine. Spine. 2004; 29(17):E376–E381.
[21] Yeom JS, Lee CK, Shin HY, Lee CS, Han CS, et al. Langerhans’ cell histiocytosis of the spine: Analysis of twenty-three cases. Spine. 1999; 24(16):1740–1749.
[22] Gogia N, Marwaha V, Atri S, Gulati M, Gupta R. Fibrous dysplasia localized to spine: A diagnostic dilemma. Skeletal Radiol. 2007; 36(1):S19–S23.
[23] Smith SE, Murphey MD, Motamedi K, Mulligan ME, Resnik CS, et al. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. Radiographics. 2002; 22(5):1191–1216.