Full Text
Once upon a time contraception was a taboo in many countries and practicing or even promoting contraception was punishable. From that situation oral pills have now reached a stage where they are most widely accepted method of birth control and also became a useful therapeutic modality for many gynaecological conditions avoiding unnecessary hysterectomies. Many breakthroughs in the evolution of birth pills are responsible for today's high acceptance of oral pills in fertility control and their other clinical uses and benefits.
History and evolution
Since ages human beings have attempted to invent and use contraception. In the past oral contraception was practiced with herbs, roots, minerals and oils. Oral pill is a major breakthrough in the field of contraception.
even though the passion of Margaret Sanger, the money of Katherine McCormi, the pharmacological genius of Gregory Pincus and the clinical responsibility of John Rock resulted in the invention of oral contraceptive pill, Gregory Pincus (Figure 1) name is associated with oral pill. First pill was developed in 1961. Since then several stepwise changes in dosage and composition of pill have taken place in order to get a safe and effective pill.

Milestones
In 1921, Ludwig Haberlandt, grandfather of pill found that rabbits and guinea pigs became temporarily sterile after transplantation of ovaries from pregnant animals this finding led to the study of the effect of progesterone on ovulation. In 1937, A. W. Makepeace and co- workers demonstrated the anti-ovulatory effect of progesterone. Russell Marker, organic chemistry professor found that progesterone can be synthesized from a substance “diosgenin” extracted from the root of plant Dioscorea mexicana. Gregory Pincus along with Min Chueh Chang found that repeated injections of progesterone stopped ovulation in animals. Carl Djerassi (syntex company) synthesized the first orally active progestin norethindrone.
Frank Colton (Searle) developed close isomer of norethindrone namely norethinodrel. First oral contraceptive trials were started with norethinodrel. Subsequently it was found that addition of a synthetic esrogen mestranol stops the breakthrough bleeding.
Thus in 1957 first contraceptive pill “enovid” containing 10mg of norethinodrel and 150mg of mestranol was developed by Searle Company. First it was approved by FDA only for the treatment of menstrual disorders and later it got approved as contraceptive in 1961. However Searle did not market enovid 10mg as contraceptive pill as they developed low dose enovid with 5/2.5mg of norethinodrel and 75mg of mestranol, which is equally effective and got approval in 1961 for oral contraception in early 60s Schering developed oralpill containing 4mg of norethisterone and 50mg of ethynylestradiol (anovlar).
Organon developed “lyndiol” (2.5 mg of lynestrenol & 75mg of mestranol). Ethinyl estradiol was synthesized by Hans Herloff Inhoffen and Walter Hohlweg in 1938 and later it replaced mestranol in all OC pills. Joseph Goldzieher was first to develop sequential pill in which oestrogen will be given for 2 weeks followed by oestrogen along with progesterone, for one week. Pills with natural oestrogens have been developed very recently.
Knowledge of the fact that high doses of sex steroids particularly ethnylestradiol are responsible for the serious side effects of pills, led to the development of newer pills with low dose oestrogen, newer progestins with more specific action and alternative administrative routes and schemes . This resulted in the development of combined pills & phasic pills. Due to dose reduction, extension of pill intake to 24 days became necessary to ensure sufficient suppression of follicular development. Only in the recent past, the extended regimens are promoted. Besides, ‘progesterone only’ pills have also been developed for use in women in whom oestrogen use is contraindicated. Even though the dose reduction resulted in reduction of side effects like nausea, breast tenderness, vomiting, and bloating, the pro thrombotic effect is not nullified. The effect of progestin on endometrium even in low dose is dominant.
The newer progestins are 2nd generation (levonorgestrel); 3rd generation (gestodene and desogestrel); 4th generation (drospirinone, dienogest & normogestrol); Natural estradiol - attempts to replace ethynylestradiol with natural oestrogen (estradiol) resulted in the development of a four phasic pill, a combination of estradiolvalerate which is cleaved into estriol and dienogest in an extended cycle of 26/28 days.
A second OC containing 17 alpha estradiol and normogestrol acetate 24/4 regimen is about to be launched. In 1980 low dose pills containing 0.015 -0.025 mg of ethinyl estradiol are developed, which are frequently used now. Research currently going on to develop non-steroidal pills, pills that can be used only in post ovulatory phase or following coitus and pills that can be used less frequently - may be once in a month.
Classification of steroidal contraceptives
Estrogen and/or progesterone administration has been tried through various routes in order to provide a reliable contraceptive cover. Various types of available steroidal contraceptives are shown in figure 2.
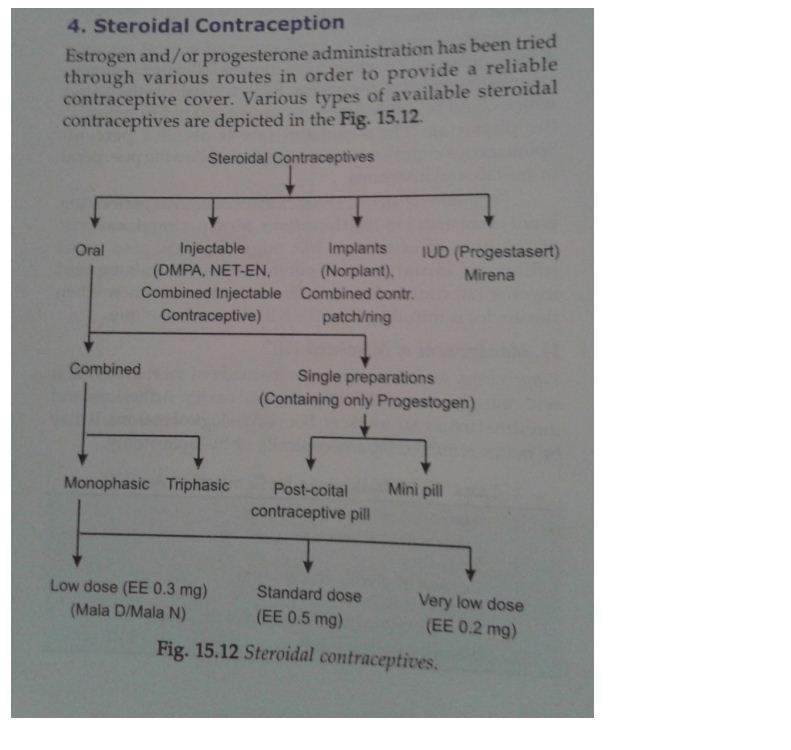
Classification
Depending upon the amount of oestrogen and type of progesterone, pills are defined as
1st generation with 50µg of ethinyl estradiol and progestin noretynodrel, 2nd generation with 20-35µg of ethinyl estradiol and progestin levonorgestrel/norgestimate
3rd generation with 20-30µg of ethinyl estradiol and progestin desogestrel/ gestodine
4th generation with 20-30µg of ethinyl estradiol and progestin drosperenone/ dienorgest/ normegestrol.
Oral pills
Oral pills are broadly divided in to two groups: 1) combined pill, and 2) progesterone only pill or mini pill. Combined pills are of two types - monophasic or multiphasic. Monophasic pills contain oestrogen and progestin in the same amount in each pill. They are again divided into a) low dose pills containing ethinyl estradiol less than 0.05mg and very low dose pills with ethinyl estradiol less than 0.015-0.025 mg in each pill along with progestins.
Multiphasic pills
With a view to reduce total monthly dose of steroids, phasic pills have been introduced. They contain low doses and variable amount of oestrogen and progestogen in 2 (biphasic) or 3 (triphasic) periods with in a menstrual cycle. The dose of progestin is low at the beginning and high at the end, while oestrogen dose is constant or slightly rises in mid cycle. Biphasic pill contains 0.035mg of ethinyl estradiol and low dose progestogen for 10 days and higher dose of progestogen for the following 11 days, not popular because of higher failure rates. They are not available in India. Triphasic pills in India contain 0.05mg of l-norgestrel and 0.03 mg of ethinyl estradiol per day for the 1st 6 days, 0.075mg of l-norgestrel and 0.03 mg of ethinyl estradiol per day next 5 days and 0.125mg of l-norgesrel and 0.03 mg of ethinyl estradiol per day during last 10 days (triquilar). Triphasic pills with newer progestogens are available in other countries.
Mini pill or ‘progesterone only’ pill
The ‘progesterone only’ pill contains only progestrogen in the form of norethisterone (0.0350 mg). This quantity is 50% of that of combined pills. They are available in India by name zerogen. It is suitable for lactating mother and in women in whom oestrogen is contraindicated.
Extended regimens
Monophasic pills prescribed continuously during 42 (bi) or 63 (three cycling) days.
FDA approved a pre-packaged extended cycle regimen 84/7 containing 30mg ethinyl estradiol along with 0.15mg of l-norgestrel. Other brands like Seasonique and LoSeasonique use a different configuration of hormones. Both of these pills use oestrogen in the final week with LoSeasonique providing low dose option. In 2007 an annual regimen of extended cycle was approved by FDA. It contains 20 µg of ethinyl estradiol along with 90 µg of l-norgestrel to be taken throughout the year.
Newer oral contraceptives
Nupil (noperalate): Chewable pill. Ovacon 35; contains progesterone (norethindrone) and oestrogen ethinyl estradiol. It is available as 28 day regimen. After chewing the pill they have to take 8-oz of water.
Alternative routes and administration
These include hormone releasing intrauterine contraceptive device (IUCD), injectable contraceptives, implants, vaginal rings and dermal patches. Vaginal rings and dermal patches came in to market in 2001. Parenteral route has got the advantage of bypassing the 1st pass effect. There is no evidence that these new modalities of administration are superior to OC pills.
Mechanism of action
Inhibition of ovulation by suppressing the hypothalamo-pituitary axis, thickens the cervical mucus thereby preventing sperm migration through cervix, makes endometrium less receptive for implantation.
Benefits
One of the most effective method of contraception with a pregnancy rate of 1/1000 women in 1st year better than any other reversible contraceptive method except injections and implants; In addition to cycle stabilization, withdrawal bleeding is predictable, which can be postponed safely by means of extended regimens; Menstrual disorders such as cycle abnormalities, dysmenorrhoea, ovulation pain can be controlled by using low dose pills (IPPF, 2003). Protection against cancer is one of the most important benefits of OCPs. It has been proved that they prevent endometrial cancer by 50% (IMAP) and effect lasts for about 15 years (Cash,1987a) with one year of usage and both high and low dose pills prevent ovarian cancers by 40%, effect persist for 10 years (Cash, 1987b). They indirectly prevent choriocarcinoma by preventing pregnancy; Protection against benign breast diseases- fibroadenoma and fibrocystic diseases of breast are reduced by 50% in pill users. Protection increases with duration of usage and dose of progesterone; The risk of follicular cyst is reduced to 50% and that of corpus luteum by 80% (WHO, 1996); Uterine fibroids - oxford family planning association has shown in their long term study that the risk uterine fibroid is reduced by about 30% with 10 year usage of OCPs. Protection is proportional to duration of use. Low dose pills also reduce fibroids and menstrual bleeding (WHO, 1996; IMAP, 2002, 2003); Protection against ectopic pregnancy (50%) and pelvic inflammatory disease (50%). Combined pills can be used continuously to control endometriosis; Low dose pills with newer progestogens are effective in controlling acni and hirsutism by reducing sex hormone binding globulin and significantly increasing free testosterone levels; Rheumatoid arthritis - pills have a protective effect in the development of rheumatoid arthritis and may reduce progression from mild to severe stage of the disease (Speroff and Darney 1996); Some reports show that low dose pills reduce the chance and degree of osteoporosis (IPPF, 2003); No effect on future fertility - ovulation usually returns in 3 months after stopping the pill (Tindall, 1987)
Side effects
Minor side effects include nausea, vomiting, loss of appetite, breakthrough bleeding. Low dose pills may cause oligomenorrhoea/menorrhagia or irregular bleeding due to either low oestrogen or progestogen resulting in lack of proliferation or regression of endometrium. Amenorrhoea is usually temporary and can be treated with changing to triphasic pills or by supplementing with ethinyl estradiol 0.02 mg in last 7 days for few cycles (Wentz, 1998). If not responding OCP should be stopped. Post pill amenorrhoea may occur in long term users and can be treated with cyclical treatment with ethinyl estradiol 0.05mg for 10 days every 4 weeks for few cycles. However 1% of long term users of pills, who have menstrual problems before the use of pill (Tindall, 1987), may have persistent amenorrhoea and they may require investigations for micro adenoma of pituitary. They may respond to treatment with clomiphene and rarely treatment with bromocryptine is needed. More often this side effect is cured by cyclical oestrogen.
Other minor effects include breast tenderness, heaviness, vaginal discharge, headache, migraine, chloasma, weight gain, acni, psycho sexual troubles, corneal edema and hyperlipidaemia. The last two may require withdrawal of pills.
Major side effects and risks
The risks of low dose pills are much less than high dose pills. The information on the long term effects of low dose pills is not yet available. Coagulation and lipids - cardiovascular complications in OCP users may be mainly due to alteration in coagulation system brought about by oestrogen component. Progestogens are associated with the increase of low- density lipoprotein cholesterol and a decrease of high – density cholesterol, which enhance the risk of atherosclerosis, coronary heart disease and cerebral thrombosis: but oestrogens have the opposite effect, and these actions seem relatively balanced in low- dose pills. Todate, there are no data to suggest that low- dose OCP have clinically significant adverse effect on lipid profile.
Cardiovascular effects
There is no increased risk of MI in non-smoking women without hypertension (HTN) and diabetes mellitus (DM) with the usage of low dose pills, irrespective of their age and duration of the use of pill (WHO, 1998). The suggestion that there is less risk of myocardial infarction with low dose pills containing Desogestrel or Gestodene than LNG is not yet proved.
Stroke
The increased relative risk of ischemic stroke is about 1.5 times with low dose pills and the risk is more in smokers and 3 times more in women with hypertension (WHO, 1998; IMAP 2002).
Venous thromboembolism
The risk of venous thromboembolism is 3 to 6 times more in pill users and rises with increasing age, recent surgery, and some forms off thrombophilia. OC pills containing desogestrel and gestodone may carry a slightly greater risk of venous thromboembolism (1.6 fold) than OCP containing l-norgestrel.
Hypertension
In women above 35 years OCP use may cause mild hypertension. This may be the effect of progestogen component. Hypertension usually disappears on discontinuation of pills.
Breast cancer
The WHO researchers state that in young women there is a small risk of breast cancer(1.24) which reduces gradually and disappears completely 10 years after discontinuing the pill (WHO,1996).
Cervical cancer
There is a modest increase in the risk of cervical cancer (1.3 – 1.8 f old) among women who have used OCP for more than 5 years. However it is not clear whether the risk is due to direct effect of the pill or due to other factors such as age at 1st inter course, multiple partners, parity smoking etc. long term use of OCP may be a co-factor in increasing the cervical cancer risk in women with persistent hypertension. The highest excess (four fold) cancer risk was seen in women who used OCP for 10 years or more. At present it is acceptable to continue OCP while monitoring or treating CIN (Guillebaud, 1999; WHO 2002).
Liver cancer
The risk of liver cancer is very small in OCP users, the risk may be slightly higher in developed countries where hepatitis infection is more common. WHO study (1992) did not find an increased risk of liver cancer with short term use of OCP, even in areas where Hepatitis B infection is more prevalent.
Diabetes
There is no evidence of increased incidence of diabetes in OC users. (IPPF, 1998). Low dose OCPs can be used in Diabetic patients under medical supervision. The insulin dose may have to be increased slightly. BP to be monitored as hypertension in these cases substantially increases the risk of arterial disease (1998).
Effect on liver and gallbladder
OCPs may cause cholestasis and cholestatic Jaundice occasionally. There is an increased risk of gallstones and gallbladder disease in pill users, however acceleration of these effects occurs only in those women who are susceptible to them in any case, and no long-term increased risk has been found (IMAP, 2002).
OCPs increase incidence of rare benign liver tumour- primary hepatocellular adenoma (1.2 per 100,000 women (Wentz, 1988), but no risk of liver neoplasia in short term users (WHO, 1992).
Effect on lactation: OCP use will reduce milk production (WHO, 1983).
Teratogenicity and future pregnancies
OCP use in early pregnancy is not associated with the increased incidence of major fetal malformations or spontaneous abortions (IPPF, 1987; population reports, 1990).
Nutritional disorders
Studies of women with borderline malnutrition have shown little or no adverse effects of OCs on Nutritional status (WHO 1980), There is no need of vitamin supplementation in pill users (IPPF, 1987).
Conclusion
The benefits of oral pills far outweigh the risks, more so in Indian women, who often suffer with anaemia, malnutrition and unwanted pregnancies. The risks of voluntary pregnancy termination are much higher than risks from pill. In cases where there are no contraindications, OCPs are practically harmless. They are less hazardous than common activities like games, motoring, sailing, and certainly less hazardous than smoking. Still the quest for achieving ideal OCPs, which is convenient for the present day working women to accept, without any side effects is going on. Research should be focussed on sustained release oral pill, which can be administered once in a month so that patients compliance can be improved with desired effect.
Conflicts of interest
Authors declare no conflict of interest
References
1. Adi ED, Tank PD. The Oral Contraceptive Pill: The early days of a 50 year-old legend. J Obstet Gynaecol India. 2010; 60(3): 207–209.
2. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003; 68(2):89–96.
3. Chaudhuri SK. Editorial column. J Indian Med Ass. 1986; 84:169.
4. Fortney JR, Harper JM, Potts M. Stud Fam Plan. 1986; 17(3):117.
5. Atkinson LE, Lincoln R, Forrest JD. Int fam plan perspect. 1985; 1(4):100.
6. Back DJ, Brickenridge AM, Orme MLE. IPPF Med Bull. 1983; 17(1):1.
7. Birtch RL, Olatunbosun OA, Pierson RA. Ovarian follicular dynamics during conventional vs. continuous oral contraceptive use. Contraception. 2006; 73(3):235–243.
8. Dhont M. History of oral contraception. Eur J Contracept Reprod Health Care. 2010;15 Suppl 2:S12–8.
9. Guillebaud J. In: London N, ed. Handbook of family planning. Edinburg: Charchill Livingstone, 1991.
10. Chaudhury SK. Practice of fertility control: A Comprehensive Manual, 7th edition 2013.
11. Eyong E. IPPF Med Bull Apr. 1987; 21:4.
12. CASH: Cancer and steroid hormone study. J Am Med Ass. 1987a; 257:786.
13. CASH: Cancer and steroid hormone study. N Engl J Med. 1987b; 316: 650.
14. Cohen J. IPPF Med Bull Aug 1985; 18(4)1.
15. Coney P, Washenik K, Langley RG, et al. Weight changes and adverse event incidence with a low-dose oral contraceptive. Contraception 2001; 63:297–302.
16. Conly SR, Camp SL, eds. India’s family planning challenge: From rhetoric to action, countryside series 2. Washington: Population crisis committee, 1992.