Case Report
2022
December
Volume : 10
Issue : 4
Pierre robin sequence with patent ductus arteriosus: A case report
Srimathi T
Pdf Page Numbers :- 248-250
Srimathi T1,*
1Department of Anatomy, Sri Ramachandra Institute of Higher Education and Research, Chennai-600116, Tamil Nadu, India
*Corresponding author: Dr. Srimathi T, MD, Department of Anatomy, Sri Ramachandra Institute of Higher Education and Research, Chennai-600116, Tamil Nadu, India. Email: drtsanatsrmc@gmail.com
Received 15 July 2022; Revised 3 September 2022; Accepted 17 September 2022; Published 23 September 2022
Citation: Srimathi T. Pierre robin sequence with patent ductus arteriosus: A case report. J Med Sci Res. 2022; 10(4):248-250. DOI: http://dx.doi.org/10.17727/JMSR.2022/10-46
Copyright: © 2022 Srimathi T. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
When Pierre Robin sequence is associated with many congenital abnormalities, prompt diagnosis and treatment are essential. As Pierre robin sequence can cause significant upper respiratory tract obstruction and aspiration pneumonia it needs detailed evaluation and early correction. Here we report a two years-old girl child with patent ductus arteriosus associated with cleft palate and tongue tie (Pierre Robbin sequence) who was promptly treated. This type of presentation is rare and important. A girl child with a Pierre robin sequence, associated with PDA was under follow-up since birth. The baby was mildly tachypnoeic & was started on diuretics at one month of age. Early closure of PDA with a device was done at 11 months of age. At the age of two years, corrective surgery was done for cleft palate, and tongue tie (Pierre Robin sequence). Pierre Robin sequence may be associated with congenital heart disease, like ventricular septal defect, patent ductus arteriosus, and atrial septal defect in 20 percent of patients. If not treated, the airway obstruction can produce cardiac or respiratory failure, pulmonary hypertension, etc. Early diagnosis and timely intervention helps to alleviate the difficulties. Antenatal screening in high-risk mothers and early identification of the congenital anomaly are the only methods to detect and treat such congenital anomalies.
Keywords: congenital heart diseases; craniofacial anomalies; cleft lip; Pierre Robin sequence; cleft palate; micrognathia; retrognathia
Full Text
Introduction
A two-years-old girl child with patent ductus arteriosus associated with cleft palate and tongue tie (Pierre Robin sequence; PRS) was treated in our hospital. This type of presentation has clinical significance. Pierre Robin sequence (PRS) is named after French stomatologist Pierre Robin who first documented the disorder [1]. PRS was originally described as consisting of micrognathia and glossoptosis (i.e., an abnormal posterior placement of the tongue), which results in airway obstruction and feeding difficulties [2]. The prevalence of PRS is one in 8,500 to one in 20,000 births. In a syndrome, multiple anomalies arise from single underlying pathogenesis. However, in PRS, multiple anomalies result from a sequential chain of malformations, each one causing the next. In PRS, the micrognathia leads to glossoptosis, resulting in airway obstruction and an inability to feed [3]. In 20% of patients, congenital heart diseases, like ventricular septal defects, patent ductus arteriosus, and atrial septal defects, may be associated. The airway obstruction can produce cardiac or respiratory failure or pulmonary hypertension if not treated.
The etiology of PRS is often multifactorial. In syndromic cases it is due to gene mutation. In non syndromic cases it occurs due to intrinsic or environmental factors. Fairbairn-Robin triad (FRT) with cleft palate and the Siebold-Robin sequence (SRS) without cleft palate are the two subdivisions of PRS [4]. Both present with micrognathia and glossoptosis. Retrogenia or posterior displacement of the chin may also occur in PRS [5]. This classification helps to decide about the treatment options. Early diagnosis and timely intervention helps to alleviate the difficulties. Antenatal screening in high-risk mothers and early identification of the congenital anomaly are the only methods to detect and treat such congenital anomalies.
Case report
At two-years-old the patient presented with fever and difficulty breathing for three days. The patient was a female child with PRS associated with patent ductus arteriosus. She was under follow-up since birth. The baby was mildly tachypneic and was started on diuretics at one month old. Early transcatheter closure of PDA with a device was done at 11 months of age.
Physical examination revealed the patient was febrile on admission. Her pulse rate was 84/min, and her respiratory rate was 28/min. A cardiovascular examination revealed normal heart sounds. There was a cleft lip, cleft palate, tongue tie, and retrognathia indicative of PRS.
An echocardiogram revealed a patent ductus arteriosus with a residual shunt across the device. Normal left aortic arch, good biventricular function, no pericardial effusion (Figures 1 and 2). RT PCR was negative for COVID-19. Hematological investigations were within the normal range.
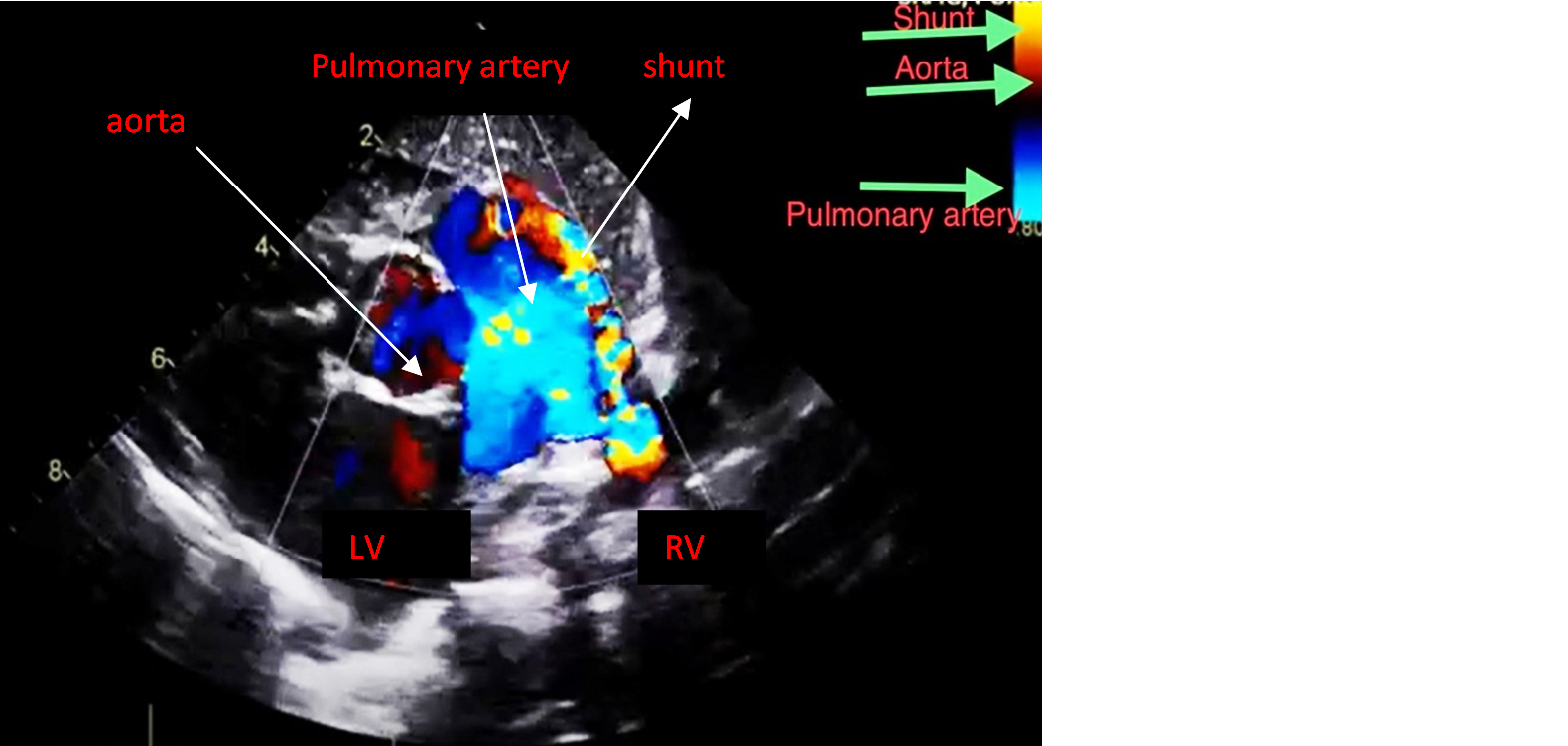
Figure 1: Echocardiogram showing the Patent ductus arteriosus.
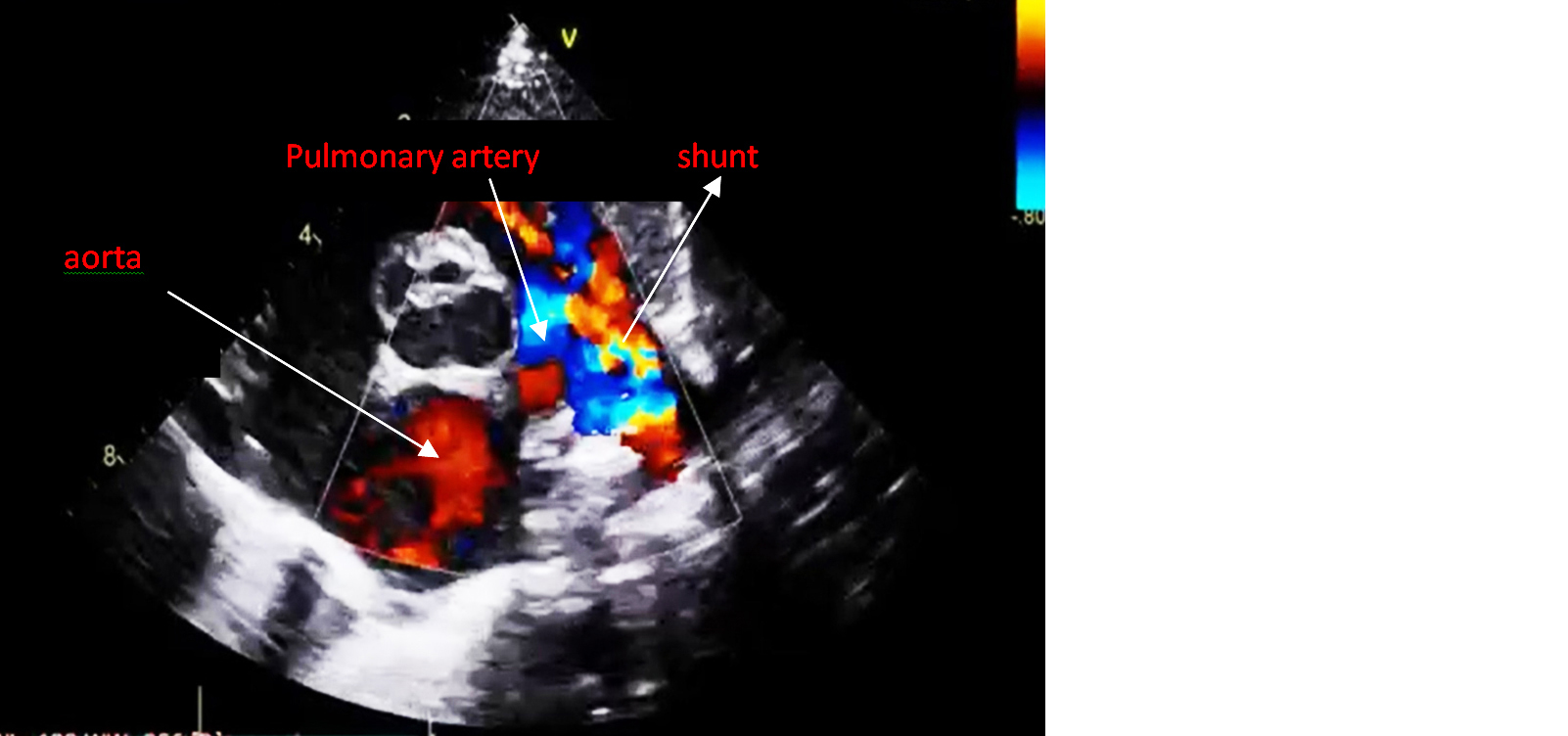
Figure 2: Echocardiogram of PDA with minimal residual shunt across the PDA device.
At two years old, the patient underwent palatoplasty for cleft palate and tongue tie release once her vitals became stable with conservative treatment. Bilateral otoendoscopy was done. Myringotomy with Grommet insertion was done to restore the function of middle ear.
Postoperatively the patient became stable and afebrile. She was discharged with advice to review in the plastic surgery department. Speech therapy was started at review.
Discussion
PRS presents with different types. One is isolated PRS, or nonsyndromic. The second is syndromic, and the third is with accompanying anomalies. There were reports of isolated cases in 48% of patients, syndromic in 35%, and accompanying other anomalies in 17%among 110 cases [6]. Previous authors reported a 40% rate of isolated cases, a 25% rate of syndromic cases, and a 35% rate of accompanying anomalies [7]. The rate of isolated PRS was reported to be 74% [8]. In nonsyndromic cases changes in the DNA near the SOX9 gene may be the causative factor. As a result, the SOX9 protein cannot control the genes that play a key role in the normal development of the mandible. Micrognathia causes discrepancy in between the tongue and oral cavity. The large tongue interferes with fusion of the palatal shelves causing cleft palate. The macroglossia causes feeding and breathing difficulties. SOX 9 gene is the positive regulator in the development of fetal mandibular cartilage. It is an enhancer in type II collagen expression and palatal cartilage development. It is also important for development of neural crest. So defects in SOX9 gene may lead to defects in the development of craniofacial structures [9].
In syndromic cases, it may be associated with (i) Stickler syndrome which is an autosomal dominant disorder due to mutations in COL2AI gene which codes for type 1 collagen. Patients present with myopia, osteoarthritis, hearing loss along with PRS. (ii) Catel-Manzke syndrome which is an autosomal recessive disorder with TGDS gene mutation. Patients present with clinodactyly and hyper phalangism. (iii) Richierie-Cota-Pierra syndrome which is an autosomal recessive disorder with EIF4A3 gene mutation. Clinical features include preaxial ray deficiency, abnormal fusion of mandible with absent lower incisors. (iv) TARP syndrome which is a X linked recessive disorder with mutation of RBM10 gene. May present with talipes equinovarus, atrial septal defect and persistent left superior vena cava [9].
Our case was PRS with an accompanying anomaly (patent ductus arteriosus). The incidence of PDA has been reported to be one in 2,000 births [10, 11]. This accounts for 5% to 10% of all congenital heart diseases. If children with “silent” patent ductus (PDA found incidentally) are also included, the incidence has been estimated to be as high as one in 500 [12]. The female-to-male ratio is 2:1 in most reports. Feeding problems can be managed by appropriate positioning of the children, prone or lateral. Nasopharyngeal airway can relieve airway obstruction. Supplemental oxygen maybe needed in case of respiratory distress. Surgical management consists of Tongue lip adhesion to correct glosoptosis. This should be done before dentition of lower teeth. The Tongue lip adhesion must be released after growth of the infant. Mccarthy advised distraction osteogenesis of the mandible to lengthen the mandible forward which automatically pulls the tongue forward relieving the airway obstruction. If not treated, the airway obstruction can produce cardiac or respiratory failure [13]. The treatment of PDA may range from use of Indomethacin in mild cases to surgical ligation in a hemodynamically significant PDA. Catheter devices can also be used for closure of PDA. PRS can be subdivided in the future into Fairbairn-Robin triad and Siebold-Robin sequence cases because the treatment approaches differ. The treatment may be nonsurgical or surgical depending on the severity of the disease.
Conclusion
The anomalies associated with PRS have a poor prognosis. Diagnosis and timely intervention using a multidisciplinary approach are the modalities of management. In our case the baby was diagnosed to have PDA with PRS and was started on diuretics at one month of age. Transcatheter closure of PDA was done at 11 months of age. At two years of age palatoplasty with tongue tie release was done which relieved the airway obstruction. Antenatal screening in high-risk mothers and early identification of the congenital anomaly are the only methods to detect and treat such congenital anomalies.
Conflicts of interest
Author declares no conflicts of interest.
Acknowledgement
Professor and Head, Department of Anatomy, Dean of Medical College, Department of Interventional Cardiology, SRIHER.
References
[1] Guyuron B, Persing EE. Plastic Surgery Indications and Practice. Cleftpalate J eds. 2009; pp.508-509.
[2] Mackay, Donald Roy. Controversies in the diagnosis and management of the Robin sequence. J Craniofacial Surgery. 2011; 22(2):415–420.
[3] Hunt JA, Hobar PC. Common craniofacial anomalies: the facial dysostoses. Plastic and Reconstructive Surgery. 2002 ; 110(7):1714–1725.
[4] Kurt-W Bütow. Pierre Robin sequence: Subdivision, data, theories, and treatment Part 1: History, subdivisions, and data. Annals of Maxillofacial Surgery. 2016; 6(1):34–36.
[5] E.T. Waters. Pierre Robin sequence and double aortic arch: a case report. Int J Pediatric Otorhinolaryngol. 2005; 69(1):105–110.
[6] Robin P. A fall of the base of the tongue considered as a new cause of nasopharyngeal respiratory impairment: Pierre Robin sequence. Plast Reconstr Surg. 1994; 93(6):1301–1303.
[7] Renault F. EMG of the face, tongue and pharynx in the infant: a method of studying disturbances of sucking and swallowing and studying their pathophysiology Improving the Use of EMG in Paediatrics. Neurophysiol Clin. 1992; 22(3):249–260.
[8] Fawer CL. Auditory brain stem response in neurologically normal preterm and full term newborn infants. Neuropediatrics. 1982; 13(4):200–206.
[9] Mohammad M. Al-Qattan, Almohrij SA. The pathogenesis of pierre robin sequence through a review of SOX9 and its interaction. Plast Reconstr Surg Glob Open. 2022; 10(4):e4241.
[10] Rogers GF, Murthy AS, LaBrie RA, Mulliken JB. The GILLS score:partI. Patient selection for tongue-lip adhesion in robin sequence. Reconstruction in craniofacial syndromes. Plast Reconstr Surg. 2011; 128(1):243–251.
[11] Bartlett SP, Losee JE, Baker SB. Reconstruction in craniofacial syndromes. Plastic Surgery. 2006; 4:514–516.
[12] Bijnen, Caroline L, Griot D, Peter JW. Tongue-lip adhesion in the treatment of Pierre Robin sequence. J Craniofac Surg. 2009; 20(2):315–320.
[13] Gangopadhyay N, Mendonca DA. Pierre robin sequence. Semin Plast Surg. 2012; 26(2):76–82.