Full Text
Introduction
Polycystic ovary syndrome (PCOS) is widely defined as an endocrine and metabolic disorder with a combination of signs and symptoms of androgen excess (hirsutism and/or hyperandrogenemia (HA)) due to excessive production by the ovaries or the adrenal cortex, alterations of the hypothalamus-pituitary-gonadal axis and dysregulation of ovarian folliculogenesis that leads to menstrual disorder and ovarian dysfunction (oligo-ovulation and/or polycystic ovarian morphology (PCOM)) [1]. PCOS is the most common reason for female infertility these days, affecting 4 to 20% women of reproductive age [2]. In 2003, the meeting of experts in Rotterdam revised the criterion; according to the European Society for Human Reproduction and Embryology/ American Society for Reproductive Medicine (ESHRE/ASRM). PCOS is diagnosed by the presence of 2 out of the given 3 features: oligo and/or anovulation, clinical and biochemical signs of HA and PCOM. The presence of 12 or more follicles measuring 2–9 mm throughout the entire ovary or an ovarian volume = 10 cm3 defines the PCOM [3, 4]. Biochemical findings of this disease include irregular gonadotropin secretion (increased luteinizing hormone [LH] secretion, increased ratio of LH to follicle-stimulating hormone [FSH]), increased testosterone levels, decreased sex hormone-binding globulin (SHBG), and chronic low-grade inflammation [5, 6].
The etiology of PCOS is unclear; however, various factors have been identified that might be involved in generating a hormonal and metabolic imbalance that can lead to the development of this syndrome [7]. PCOS is a genetically determined disease that shows clinical and biochemical heterogeneity depending on the interaction between environmental and genetic factors [8]. The hypotheses proposed for the etiology of PCOS over the years indicates the fact that, besides gynaecological problems, and fertility related complications, PCOS can also lead to systemic metabolic disorders (such as obesity, hyperinsulinemia and insulin resistance (IR), dyslipidemia, an increased risk of type II diabetes, and cardiovascular disease) [9]. Number of evidences on the correlation between the development of metabolic disorders and the intestinal microbiome, have led to postulate the hypothesis indicating that alterations in the microbiome are also involved in the pathogenesis of PCOS [10]. In 2012, the dysbiosis of gut microbiota (DOGMA) hypothesis suggested that an increase in intestinal permeability and an imbalance of intestinal flora could cause leakage of lipopolysaccharide (LPS) in the systemic circulation and could lead to the activation of the immune system and an inflammatory response leading to IR [11]. More recent studies have also begun to consider the role of vaginal microbiome in the pathogenesis of PCOS [12].
While reviewing the literature for the role of microbiome in the genesis of different diseases lead us to exploring the evidence published on microbiota in PCOS. The main aim was to explain the relationship between PCOS and the various microbiota (the gut and vaginal microbiota). Moreover, various mechanisms of different microbiota in the pathogenesis of PCOS and applications of different microbiota in treating PCOS were also reviewed, which might be potential for the intervention of PCOS and other related metabolic and endocrine disorders.
Material and methods
Search strategy
A computerized literature search was conducted using Medical Subject Headings (MeSH) terms such as “PCOS”, “microbiome”, “molecular mechanism”, “insulin resistance”, “therapeutic strategies”, “sexual hormones” in PubMed, Web of Science, EMBASE and Scopus Database to identify potentially relevant articles of PCOS in the English language and published from inception to August 2022.
Study selection
The selection criteria for the narrative review included original articles (randomized and non-randomized clinical trials, including prospective observational studies, case-control studies, and cohort studies) and review articles regarding the influence of microbiome on PCOS. Articles that met the selection criteria were carefully read and when found appropriate, further articles were also retrieved from their references and reviewed with the aim of including other critical studies that the initial search could have missed. Figure 1 reports the article selection flow chart.
Figure 1: Flow chart of literature search.
The microbiota
In the past few years, focus on the microbiota and human health has been increased. Microbiomes are known as microorganisms that live inside the human body and play a vital role in maintaining health. The microbiota community can be defined as a complex ecosystem of microorganisms such as viruses, bacteria, fungi and protozoa. All of them are widely spread in almost all the parts of human body including mouth, gastro-enteric tract, skin, Respiratory tract, vagina, etc. Each microbiota community plays an important role in the regulation of the homeostasis of various systems through different pathways [13].
Gut microbiome
The adult human gut microbiota consists about 1013 and 1014 micro-organisms/mL of lumina contest with the total weight estimated 1.5 kg [14]. Around 1000 different kinds of species and approximately 7000 different kinds of strains are classified and named, including Viruses, Bacteria, Fungi, Protozoa and Archaea [15]. Even though the initiation and establishment processes of gut microbiota in the early stages of life are still undefined, it is widely accepted that the development of microbiota begins immediately after birth and is influenced by various factors like age, lifestyle, diet, medication, etc. The human gut microbiota is mainly divided into five bacterial phyla: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Verrucomicrobia. Firmucutes and Bacteriodetes are around 90% and Actinobacteria and Proteobacterium account for 10%, while Verrucomicrobia contain the smallest proportion [16]. Numbers of gut microbiota are reported to regulate the physiological balance in different ways including in metabolic, protective, structural integrity and histological homeostasis [17]. The disruption of the composition of intestinal microbiota (an increase or decrease in the ratio of beneficial/harmful bacteria) is associated with many diseases/ disorders. The gut microbiota plays vital roles in the metabolic process, which includes the production of vitamins, short-chain free fatty acids (acetate, butyrate, and propionate) and conjugated linoleic acid, biotransformation of bile acids, ammonia synthesis and detoxification [18]. The gut microbiota is proved to be involved in the metabolism of Butyrate and Propionate, which mediates the energy metabolism by regulating the gluconeogenesis process and cholesterol metabolism and also associated with the regulation and modulation of the immune system with anti-inflammatory, anticarcinogenic, and immunomodulatory effects [19]. According to certain research, the development of T lymphocytes may be influenced by the bacteria in the gut [20]. The status of the gut microbiota's balance is dynamic. Some basic effects on immunological functions, structural integrity and metabolic processes of the human body are associated with the gut microbiota. A better understanding of the functioning of gut microbiota would definitely lead to some important developments in therapeutics for improved health [21].
Vaginal microbiome
The vaginal microbiota has drawn attention since it has been shaped over the years by co-evolutionary processes and has been suggested being playing vital role in female health. It has been found that the majority of vaginal microbiota is dominated by the Lactobacillus bacterial species, which prevent the colonization of harmful bacteria via producing hydrogen peroxide leading to low pH values in normal situations [22]. Additionally, a variety of strictly and facultatively anaerobic microbes were discovered in healthy females, indicating that the human vagina may contain multiple microbiomes rather than just one. The vaginal microbiota is grouped in five communities: i) the CST I [dominated by Lactobacillus crispatus (L. crispatus)]; ii) CST II (dominated by L. gasseri); iii) CST III (dominated by L. iners); v) CST V (dominated by L. jensenli) and iv) CST IV (lacks Lactobacillus sp. and is extremely rich in strict anaerobic bacteria, such as Megasphera, Prevotella, Gardenella, and Sneathia) [16]. Numerous microorganisms are present in vaginal secretions, and the host feeds them nutrients for growth and development. Disruptions in vaginal microbiomes may lead to enhanced risk of gynaecological disorders/ diseases.
Microbiota composition in PCOS
Microbiota communities are in dynamic equilibrium in healthy women, disturbances in the balance of microbiota composition might be associated with various diseases. Numerous studies have shown that women with PCOS experience dysbiosis and changes in the microbiota's composition [23].
Gut microbiome and PCOS
There are significant differences found in the composition of the microbiome between healthy adults and those differences may underlie the susceptibility to various diseases [24]. The relationship between changes in gut microbiota and PCOS has been the subject of research in recent years and that has shown a significant difference in the composition of the gut microbiome in PCOS patients in comparison of healthy individuals [25–28]. α and β diversity defines changes in the microbiome: lpha (α) diversity indicates the number of species present in a community that occupy an environment in a specific community and is known as an index of the health of an ecosystem, whereas beta (β) diversity represents how one community is similar to another [23, 29].
Several studies have reported a decrease in α and β diversity in patients with PCOS [26, 27, 30, 31].
In addition to an alteration in the general composition of the microbiome, studies have also shown the same in PCOS, there is also an alteration in the balance of some species of bacteria, like Bacteroidetes and Firmicutes [25–28]. These modifications can lead to altered production of short-chain fatty acids with a negative impact on gut barrier integrity, immunity and metabolism [23]. The genus Bacteroides has an increase of Escherichia and Shigella in women with PCOS and a general gut microbiome composition similar to that of obese control women [28].
An increased abundance of Bacteroides vulgatus was reported, which was accompanied by a reduction in the levels of glycodeoxycholic and tauroursodeoxycholic acid that leads to alteration of IL-22 level [30]. The abundance of Prevotella species can also be altered; it is increased in PCOS patients, which could induce an adverse inflammatory effect on the host [32, 33]. There was a decrease in Prevotellaceae, causing a negative effect due to the loss of production of anti-inflammatory metabolites [34]. The beneficial bacteria like Lactobacilli and Bifidobacteria, which enhance the immunity and nutrient absorption, are significantly reduced in PCOS patients [35–37]. It is also found that there is a significant decrease in the abundance of Akkermensia, Lachnospiraceae and Ruminococcaceae [38]. When focused on the phylum level, it was reported the larger proportion of Actinobacteria and the smaller proportion of Bacteroidetes in PCOS women in comparison with the healthy controls [23]. It was also found that transplantation of fecal microbiota from women with PCOS would lead to an elevated proportion of disrupted ovarian functions, insulin resistance and infertility [39]. Bacteroides, are a kind of pro-inflammatory bacteria, which might be associated with the insulin resistance, hormonal disturbances and inflammation in PCOS [40] (Table 1).
The alteration in the gut microbiota associated with PCOS is unique, debatable, and not fully understood. However, several studies have tried to investigate the relationship between intestinal microbiota and PCOS; most of these have focused on the connection of intestinal bacteria with insulin resistance, regulation of the immune response, fluctuations of sex hormones and other pathological mechanisms.
Table 1: Summary of the effect of changes in gut microorganisms in PCOS patients.
|
Change in microorganisms
|
Effects
|
|
Increase of Escherichia and Shigella
|
Altered production of Short chain fatty
acids
|
|
Increase of Bacteroides vulgatus
|
Reduced levels of glycodeoxycholic and
tauroursodeoxycholic acid
|
|
Decrease of Lactobacilli and Bifidobacteria
|
Reduced immunity and nutrient absorption
|
|
Decrease of Prevotellaceae
|
Loss of production of anti-inflammatory
metabolites
|
Vaginal microbiome in PCOS
Vaginal microbiome can be altered due to Menopause, sex hormones, age and hygienic habits and even prepuberty and postmenopausal women present different kinds of microbes [41]. The main factors for the alteration of the vaginal microbiome in PCOS patients are found to be irregular menstruation and abnormal hormone levels [42]. During the normal menstrual cycle estrogen and progesterone cause periodic changes in the epidermal cells of the reproductive tract, which may play a critical role in maintaining the vaginal microenvironment and considering the irregular menstrual cycle in PCOS women, which can strongly alter the composition of the vaginal microbiome in PCOS patients. The vagino-uterine microbiome undergoes small changes in the two phases of the menstrual cycle (proliferative and secretory phase): proliferative phase seems to be related to the increase of bacterial proliferation in the vagina and endometrium, which might be the reason for the change in the reproductive tract microbiomes of PCOS patients [43–45]. The vaginal microbiome composition of PCOS women and healthy women was studied and the results were derived from 194 microbial samples that were analyzed by 16S rRNA gene sequencing, which indicated that there is a significant difference in taxa abundance between PCOS and healthy women in vaginal and cervical canal microbiomes. In PCOS women, the results show a significantly decreased composition of Lactobacillus and an increase of potential pathogenic taxa, like Gardnerella vaginalis, Chlamydia trachomatis and Prevotella [46]. A tight association was found between the onset of vaginosis, preterm labour, infertility, abortion, stillbirth, recurrent implantation failure and many other adverse pregnancy outcomes and reduced levels of Lactobacillus species in the vagina [47–50]. Gardnerella and Prevotella species are closely related to bacterial vaginosis (BV), which can be difficult to treat because of relapses and also increases women’s susceptibility to other infections [51]. Gardnerella vaginalis can also be detected in the endometrium of half of women with BV and may cause adverse effects on the procedure of embryo implantation and the growth of the fetus [52] (Table 2).
Whether the vaginal microbiome is related with the PCOS occurrence or development is still not clear. Normally, disturbance in ‘normal’ vaginal microbiome equilibrium is defined as BV, which is characterized by increasing pH, epithelial cell destruction and local inflammation and which may be linked with infertility and other adverse reproductive outcomes [53].
Table 2: Summary of the effect of changes in vaginal microorganisms in PCOS patients.
|
Change in microorganisms
|
Effects
|
|
Increase of Gardnerella and Prevotella
|
Causes bacterial vaginosis, which decreases
the probability on the procedure of embryo
implantation and growth of fetus
|
|
Increase of Chlamydia trachomatis
|
Potential pathological taxa in vagina
and cervical canal
|
|
Decrease of Lactobacilli
|
The onset of vaginosis, preterm labour,
infertility, abortion, stillbirth, recurrent implantation
failure, many other adverse pregnancy outcomes
|
Relation of microbiota in PCOS
Microbiome and insulin resistance
Insulin resistance (IR) is widely defined as an endocrine disorder, in which glucose uptake and utilization can not be increased because of the inability of insulin. Recent studies suggest that it is a metabolic disorder related to the gut microbiome [54, 55]. The gut microbiome could play a role in the development of IR; the mechanisms that connect gut microbiome, IR and PCOS were recently summarized that disturbance of gut microbiota and increasing intestinal permeability could determine a chronic low-grade inflammation by activating the immune system [56]. Proinflammatory cytokines interfere with insulin receptor function, causing IR/hyperinsulinemia; it has been founded that with the increased intestinal permeability and the consequent introduction of lipopolysaccharides into the blood circulation of humans leading to increase in fasting blood glucose and insulin levels [9, 57, 58]. Another possible relationship between the gut microbiome and IR involves gastrointestinal hormones like Ghrelin and peptide YY (PYY), which shows a negative correlation with IR and BMI and a lower level of ghrelin and PYY in women with PCOS when compared with healthy women, maybe due to increased Bacteroides species that are negatively correlated with ghrelin [28, 59, 60]. Some studies found no differences in ghrelin and PYY levels in women with PCOS compared to healthy women, however gut microbiota may cause altered secretion of these hormones leading to IR/hyperinsulinemia [61, 62]. There is some link between IR in PCOS patients and intestinal dysbiosis as the composition of the gut microbiome in patients with PCOS is significantly altered compared with that of women without PCOS. When the profile of the gut microbiome in PCOS patients with IR was investigated and compared with that of PCOS patients without IR, a significant difference was found in the gut microbiome of PCOS patients with IR and without IR [37]. Changes in the intestinal microbiota of patients of PCOS with IR that have the highest level of Bacteroidaceae and a greater decrease of Prevotellaceae compared with PCOS women without IR. Patients with IR displayed a significant difference in the abundance of Ruminococcaceae and Lachnospiraceae when compared to insulin-sensitive patients [40] (Figure 2).
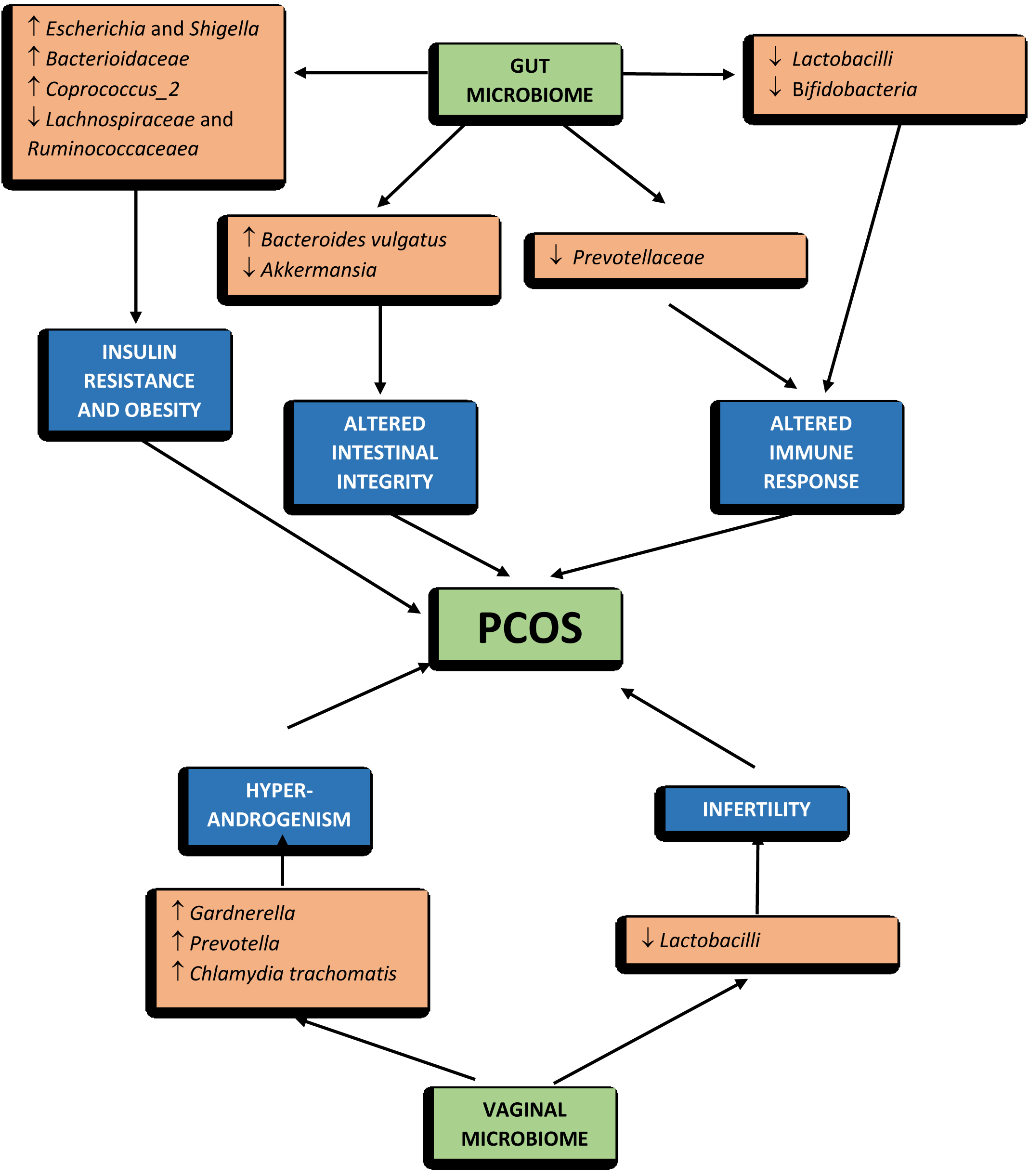
Figure 2: The microbiome changes and PCOS.
Microbiome and sexual hormones
There is evidence that gender influences the composition to that of the gut microbiome. The microbiome of women in comparison of men has greater alpha diversity and a smaller representation of Bacteroides [63, 64]. Prevotella has a strong positive correlation with testosterone and negative associations with estradiol, so were more abundant in men compared to women [65]. Men had a lower abundance of Clostridia, Methanobrevibacter and Desulfovibrio compared to women. In contrast to Megasphaera, Paraprevotella, and Butyricimonas genera, Raoultella genus is more common in women than in men. Women with PCOS have an increased abundance of the Catenibacterium and Kandleria genera [63–66]. These differences might be due to sex chromosomes or sexual hormones; while the role of sex chromosomes has not been clearly described but the latter has been extensively evaluated in studies that verified the effect of castration on the gut microbiome [67, 68]. Intestinal microbiomes can alter the sex hormones by activating the receptors in the gastrointestinal tract, altering beta-glucuronidase activity or modulating systemic/intestinal immunity [56]. A positive correlation between α diversity and testosterone levels and a negative correlation with estradiol concentrations, suggesting that sex hormones might be involved in the modification of the gut microbiome [26]. There was an alteration in gut microbiome between that of healthy controls and women with PCOS and using multiple and single linear regression analyses, the study showed an inverse correlation between the biodiversity of the microbiome and the hyperandrogenism (serum total testosterone level and hirsutism). There is a reduction in beta diversity (p = 0.006) and a modification in β diversity (p = 0.0009) [27]. The study using 16s ribosomal RNA sequencing, analyzed and compared the gut microbiome of 42 women with PCOM, 73 women with PCOS and 48 healthy women, their results highlighted a reduction in the number of bacterial species (alpha diversity) and phylogenetic diversity in women with PCOS. Women with PCOS have also shown changes in the composition of the microbial community (β diversity) [26–28]. It might be possible that hyperandrogenemia in PCOS, modifies the normal microbiome structure leading to an alteration of intestinal permeability, triggering the mechanism responsible for IR. The hyperandrogenemia promoted by IR is able to stimulate the decomposition of visceral adipose tissue, leading to an increase in free fatty acids, which further aggravate the levels of IR, promoting the occurrence and development of PCOS [69] (Figure 2). A clear correlation between hyperandrogenism and the gut microbiome in determining the pathogenesis of PCOS is still under research.
Microbiome and immune homeostasis
Numerous kinds of microbiota in human body participate in the homeostasis modification, particular in regulating the immune homeostasis [70]. There are many research focusing on the mechanism of gut microbiota in healthy human, the interactions between the gut microbiota and immune-related influence and widely reported that the intestinal immune system is mainly shaped by the gut microbiota [71]. Mainly in the intestinal immune system, myeloid cells are known as the first immune responders and the effects of gut microbiota on intestinal macrophages have been implicated [72–74]. Numerous intestinal immune defects, such as impaired development of gut-associated lymphoid tissues, gut-associated Th17 cells, decreased numbers of IgA-producing B cells, and intraepithelial CD8+ T cells, have been reported to occur in germ-free organisms [75]. A growing body of research shows that diseases in vaginal regions are linked to immune system disorders caused by the vaginal microbiota. The vaginal microbiota can also be regulated by the numerous chemical changes in the microenvironment as well as hormonal fluctuations [70]. Once the dominance of Lactobacillus is disrupted, the immune homeostasis is altered and produces pro-inflammatory cytokines, abnormal immune cell recruitment etc. [69]. The influence of the vaginal microbiota upon the female immune system is mainly to prevent external pathogen infections and to maintain an immunotolerant environment.
Pathway leading to PCOS
Though the relationship between the alteration of the gut microbiome and PCOS is not clear, only a few studies have explored the possible mechanisms through which gut microbes are associated with PCOS. Intestinal microbes influence the progression of PCOS by upregulating or downregulating hormone secretion, gut-brain mediators, cytokines, and metabolite production [69]. Relation of bile acids, interleukin-22 and Bacteroides vulgatus has been studied and reported that a significant increase in B. vulgatus in PCOS patients in comparison to healthy controls [9]. It is described that the species B. vulgatus of individuals with PCOS a significant increase in the abundance of bile salt hydrolase genes, which encode bile salt hydrolases. As a consequence, in PCOS groups, the levels of glycodeoxycholic and tauroursodeoxycholic acid decreased due to the action of the encoded protein, bile salt hydrolases leading to reduced concentrations of the bile acids, which would negatively influence the production of interleukins, normally, IL-22, which might lead to insulin resistance, regulating disrupted oestrous cycles, reversing ovary morphological changes and improving infertility, all typical aspect of PCOS phenotypes [30]. Its role may be explained through an upregulated expression of brown fat-related genes, brown adipose tissue and a resolution of inflammation targeting ovarian granulosa cells [30]. The evidence of lower concentrations of short-chain fatty acids (SCFA) in the faecal samples of PCOS patients are found [76]. Faecalibacterium prausnitzii, Bifidobacterium and Akkermansia are SCFA-producing bacteria, whose growth is promoted by probiotics and leads to an increase in intestinal SCFAs. These SCFAs bind to their receptors on enteroendocrine cell membranes and stimulate the release of gut-brain mediators such as ghrelin and PYY, which leads to sex hormone secretion by the hypophysis and hypothalamus through the gut-brain axis, thus causing the PCOS symptoms [77]. The increased levels of SCFAs affect the barrier of the gut and reduce the translocation of endotoxins across the gut wall, leading to inflammation and insulin resistance and the interaction between sex hormones and gut microbiota may contribute to the pathogenesis of PCOS [36]. The abundance of Prevotella is positively related to increased testosterone levels and the abundance of Kandleria is correlated with the circulating androstenedione concentrations [25, 78]. Lactobacilli are also positively correlated with oestradiol and estrogen levels [36] (Figure 3).

Figure 3: Pathway of the gut dysbiosis and polycystic ovary syndrome (PCOS).
Change in microbiome: Medication and diet
Since the relationship between gut and vaginal microbiome and PCOS pathogenesis is being more thoroughly researched, a link between diet, microbiota and PCOS could be postulated, which can reveal the potential impact on prevention and treatment of PCOS. Diet could play a therapeutic role for several chronic diseases, especially those related to metabolic syndrome, through modulating the microbiome. The microbiome and its modification through diet, with the influence on inflammatory bowel disease, obesity, type 2 diabetes, cardiovascular disease, cancer and its response immunotherapy [79]. The animal-based proteins have been noted to increase Bacteroides, Alistipes and Bilophila and to decrease counts of Bifidobacterium adolescentis; those changes increased the risk of cardiovascular disease & plant-based proteins have been reported to increase Bifidobacterium and Lactobacillus and to decrease Bacteroides fragilis and Clostridium perfringens, leading to a positive health outcome by increasing SCFA’s levels and decreasing inflammation. A high-fat diet increases counts of Bacteroides and correlates with less Lactobacillus intestinalis and more Clostridiales, Bacteroides and Enterobacteriales, which are associated with inflammation. The high levels of glucose, fructose, and sucrose increase Bifidobacteria and reduce Bacteroides and non-digestible carbohydrates, such as whole grain and wheat bran are linked to an increase in Bifidobacteria and Lactobacilli [79]. These dietary patterns can modify the natural history of pathologic conditions, like type 2 diabetes, with a significant reduction of glucose and improvement of dyslipidemia and inflammation, independent of antidiabetic drugs. A diet as poor as that of the “fast-food culture” can cause dysbiosis, a condition linked with, obesity, cardiovascular diseases, and cancer [80, 81]. The ability to change the microbial composition of the body through diet may have significant therapeutic value for a number of pathologies. However, only one study has looked at the potential therapeutic or preventive effects of diet on PCOS. Actually, diet and medication can contribute to potential therapy, but their protective role in the disease’s development and progression is poorly understood currently and should be further investigated.
Therapeutic opportunities of microbiome
A better understanding of the role of the microbiome in PCOS pathogenesis will lead to the development of new treatment options for the better management of PCOS.
Probiotics, prebiotics and synbiotics
Probiotics are "live microorganisms that, if supplied in adequate proportions, impart a health benefit on the host," according to the World Health Organization (WHO) [82]. Probiotic microorganisms perform antioxygenic and anti-microbial effects, anti-inflammatory effects, improving metabolic parameters, modulating intestinal microbiota, and regulating the immune system and naturally found in fermented food [83]. It was reported that probiotic supplementation of L. acidophilus, L. casei and B. bifidum for 12 weeks caused a significant decrease in weight and BMI in PCOS patients compared with the placebo along with beneficial effects on glycaemia, triglycerides (TG) and very-low-density lipoprotein (VLDL) cholesterol [83]. Lactobacillus given to letrozole-treated rats reduced androgen levels, improved estrous cyclicity, normalized ovarian morphology, increased Lactobacillus and Clostridium species and decreased Prevotella [36]. Some strains of Lactobacillus and Bifidobacterium (HL2 and HB3) given to letrozole-treated rats protected against pathological changes in the ovaries, restored testosterone levels and upregulated the levels of SCFAs [84]. A few studies also investigated the effect of the probiotic combinations Bifidobacterium, Lactobacillus acidophilus, and Enterococcus faecalis on PCOS, which improved reproductive and metabolic functions and alpha diversity of the gut microbiome [85]. The supplementation of L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, and Streptococcus thermophiles in women with PCOS for 8 weeks resulted in a significant decrease in plasma glucose and serum insulin levels and supplementation of L. delbruekii and L. fermentum for 12 weeks significantly reduced Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) levels, with an additional improvement of lipid profile [86]. The probiotic therapy with L. acidophilus, L. plantarum, L. fermentum and L. gasseri has also been reported to play a possible role in modulation of the inflammatory response when administered for 12 weeks in women with PCOS [87]. In a meta-analysis of seven RCTs no significant effect of probiotic supplementation on weight, BMI, and waist circumferences as well as HOMA-IR and LDL was seen in PCOS patients compared to placebo; instead, they found a significant effect on glycemic control, lower insulin levels and lipid metabolism, by lowering serum TG and increasing HDL levels [88]. Probiotics have been shown to have a significant impact on the regulation of hormonal and inflammatory indicators, with a significant decrease in the Free Androgen Index (FAI) and malondialdehyde (MDA), and an increase in Sex Hormone Binding Globulin (SHBG) and nitric oxide (NO) [89]. Consuming probiotic strains of bacteria has the potential to improve gut dysbiosis either directly through repopulation of the gut with healthy microbes or indirectly through the production of gut metabolites. Bifidobacterium lactis V9 given as a 10-week treatment for PCOS in 14 women with the disorder decreased LH and increased intestinal SCFAs [31]. The well-known prebiotics are fructooligosaccharides (FOS), galactooligosaccharides (GOS), inulin and lactulose; they alter the composition of the microbiota and show positive effects on health [82]. Resistant dextrin, a glucose polysaccharide that is fermented in the colon by microbes rather than absorbed in the small intestine, was given to women with PCOS and women without the disorder for 3 months which lowered levels of free testosterone, hirsutism, the interval between menstrual cycles, fasting blood glucose and lipid profile, but changes in alpha diversity were not studied [90]. Further studies are required for investigation of the effects of fermentable dietary fiber on PCOS are warranted.
Additional research is needed to elucidate and compare the efficacy of different probiotics and their doses, to establish the duration of treatment and to determine the possible health benefits of prebiotics, probiotics and synbiotics.
Fecal microbial transplant (FMT)
Since gut dysbiosis has been proposed as a driver of PCOS symptoms, although there are few studies to support this theory, treatment with an FMT from healthy donors or microorganisms from a healthy gut may serve as a viable therapeutic to re-diversify the gut microbiota. According to one study, FMT from a healthy individual into a PCOS one, caused by letrozole which reduces androgen levels, improves estrous cycles, normalizes ovarian morphology, increases levels of Lactobacillus and Clostridium species and decreases Prevotella [36]. Overall, these studies show promise that adjusting the gut microbial community in women with PCOS could change some of the diet-independent symptoms.
Small molecules: Metformin and IL22
Metformin decreases total testosterone, hirsutism, acne, LH, BMI, waist-to-hip ratio and fasting insulin, and increases SHBG, follicle-stimulating hormone, and progesterone, while also improving menstrual cycles, in addition inhibiting androgen biosynthesis [91]. Although no studies have demonstrated the effect of metformin on the gut microbiome of PCOS women. A study in DHEA treated individuals showed that it improved dysbiosis of the gut, including increased levels of Bacteroidetes and decreased levels of Helicobacter and Proteobacteria; metformin also decreased testosterone levels while also improving ovarian function, weight gain, and IR [92].
Future perspectives Most of the current studies have proposed that dysbiosis of the microbiota might play a role in the development of PCOS. Diverse aspects of PCOS like obesity, insulin resistance, androgens, microbiota, the gut-brain axis, etc have been studied, yet only a small portion of information about the underlying mechanisms have been identified. The correlation between microbial species and metabolites, with the help of metagenomic sequencing would provide a clear picture of the interactions occurring in PCOS women. The majority of data mainly obtained from cross-sectional studies are insufficient, and to elucidate their exact role in the pathogenesis of PCOS, further randomized control studies are required with strict inclusion/exclusion criteria, larger sample sizes and proper sampling procedures. Probiotic, prebiotic and synbiotic supplementation in PCOS women has shown an improvement in many biochemical findings and beneficiary effects, but the mechanism is still not clearly identified, so it requires further clinical trials to confirm the efficacy of the same for the better management of PCOS patients.
Conclusion
Though there are increasing evidence to demonstrate the correlation between the gut and vaginal microbiota and PCOS, most of the studies only draw the conclusion that the diversity of microbiota changes in PCOS women. The low level of α diversity, β diversity and Lactobacillus in PCOS, and the high level of Chlamydia trachomatis and Prevotella are seen in PCOS patients. This dysbiosis of the microbiome leads to insulin resistance, obesity, altered immune responses and hormonal disturbances which lead to ovarian dysfunction and PCOS. The contribution of gut microbiota composition and disruption in secondary bile acid biosynthesis in the pathogenesis of PCOS seems to be proven, and administration of IL-22 and glycodeoxycholic acid improved insulin resistance, ovarian dysfunction and infertility in PCOS. Shaping the gut microbiota using probiotic therapy has shown to improve the complications related with PCOS, so it is possible to believe that a future therapeutic approach for PCOS may involve mechanisms related to gut microbiota.
Conflicts of interest
The authors declare no conflict of interest.
References
[1] Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018; 132:321–336.
[2] Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health. 2018; 15:2589.
[3] Fauser BCJM. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81:19–25.
[4] Giampaolino P, della Corte L, de Rosa N, Mercorio A, Bruzzese D, et al. Ovarian volume and PCOS: a controversial issue. Gynecol Endocrinol. 2018; 34:229–232.
[5] Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004; 18:671–683.
[6] Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005; 352:1223–1236.
[7] Giampaolino P, Corte LD, Rosa ND, Mercorio A, Bifulco G, et al. Ovarian volume and PCOS: a controversial issue. Gynecol Endocrino. 2018; 34:229–232.
[8] Franks S, McCarthy MI, Hardy K, Skakkebæk NE, Aitken RJ, et al. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006; 29:278–285.
[9] Li Y, Chen C, Ma Y, Xiao J, Luo G, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019; 228:167–75.
[10] Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019; 129:4050–4057.
[11] Tremellen K, Pearce K. Dysbiosis of gut microbiota (DOGMA)--a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses. 2012; 79:104–112.
[12] Hong X, Qin P, Huang K, Ding X, Ma J, et al. Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin Endocrinol (Oxf); 2020; 93:52–60.
[13] Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. 2019; 27:997–1010.
[14] Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, et al. The gut microbiota and host health: a new clinical frontier; 2016; 65:330–339.
[15] Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020; 69:2232–2243.
[16] Gu Y, Zhou G, Zhou F, Li Y, Wu Q, et al. Gut and Vaginal Microbiomes in PCOS: Implications for Women’s Health. Front Endocrinol (Lausanne). 2022; 13:808508.
[17] Raftery AL, Tsantikos E, Harris NL, Hibbs ML. Links between inflammatory bowel disease and chronic obstructive pulmonary disease. Front Immunol. 2020; 11:2144.
[18] Zhao S, Jang C, Liu J, Uehara K, Gilbert M, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020; 579:586–591.
[19] Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019; 68:1430–1438.
[20] Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017; 46:562–576. [21] Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019; 76:473–493.
[22] Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018; 34:451–455.
[23] Thackray VG. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019; 30:54–65.
[24] Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLoS One. 2017; 12:e0168390.
[25] Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017; 474:1823–1836.
[26] Insenser M, Murri M, del Campo R, Martínez-García MÁ, Fernández-Durán E, et al. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018; 103:2552–2562.
[27] Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, et al. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J Clin Endocrinol Metab. 2018; 103:1502–1511.
[28] Liu R, Zhang C, Shi Y, Zhang F, Li L, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017; 8:324.
[29] He F, Li Y. The gut microbial composition in polycystic ovary syndrome with insulin resistance: findings from a normal‐weight population. J Ovarian Res. 2021; 14:50.
[30] Qi X, Yun C, Sun L, Xia J, Wu Q, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019; 25:1225–1233.
[31] Zhou L, Ni Z, Cheng W, Yu J, Sun S, et al. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr Connect. 2020; 9:63–73.
[32] Lukens JR, Gurung P, Vogel P, Johnson GR, Carter RA, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014; 516:246–249.
[33] Ley RE. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016; 13:69.
[34] Zeng B, Lai Z, Sun L, Zhang Z, Yang J, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019; 170:43–52.
[35] Jiao N, Baker SS, Nugent CA, Tsompana M, Cai L, et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics. 2018; 50:244–254.
[36] Guo Y, Qi Y, Yang X, Zhao L, Wen S, et al. Association between polycystic ovary syndrome and gut microbiota. PLoS One. 2016; 11:e0153196.
[37] Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018; 9:400–421.
[38] Eyupoglu ND, Ergunay K, Acikgoz A, Akyon Y, Yilmaz E, et al. Gut microbiota and oral contraceptive use in overweight and obese patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2020; 105:600.
[39] Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020; 502:214–221.
[40] Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016; 535:376–381.
[41] Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016; 91:42–50.
[42] Stumpf RM, Wilson BA, Rivera A, Yildirim S, Yeoman CJ, et al. The primate vaginal microbiome: comparative context and implications for human health and disease. Am J Phys Anthropol. 2013; 152:119–134.
[43] Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012; 4:132ra52.
[44] Chen C, Song X, Wei W, Zhong H, Dai J, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017; 8:875.
[45] Anahtar MN, Gootenberg DB, Mitchell CM, Kwon DS. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe. 2018; 23:159–168.
[46] Mehta O, Ghosh TS, Kothidar A, Gowtham MR, Mitra R, et al. Vaginal microbiome of pregnant indian women: insights into the genome of dominant lactobacillus species. Microb Ecol. 2020; 80:487–499.
[47] Gupta S, Kakkar V, Bhushan I. Crosstalk between vaginal microbiome and female health: a review. Microb Path. 2019; 136:103696.
[48] Al-Memar M, Bobdiwala S, Fourie H, Mannino R, Lee YS, et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG. 2020; 127:264–274.
[49] Peelen MJ, Luef BM, Lamont RF, de Milliano I, Jensen JS, et al. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta. 2019; 79:30–39.
[50] Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod. 2019; 34:1042–1054.
[51] Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020; 245:143–148.
[52] Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020; 9.
[53] Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016; 29:223–238.
[54] Bäckhed F, Ding H, Wang T, Hooper L v., Gou YK, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. National Academy of Sciences; 2004; 101:15718–15723.
[55] Scheithauer TPM, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016; 5:759.
[56] He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020; 13:73.
[57] Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cell Physiol Biochem. 2015; 37:1029–1043.
[58] Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RMG, Mller K, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010; 104:1831–1838.
[59] Mitkov M, Pehlivanov B, Orbetzova M. Serum ghrelin level in women with polycystic ovary syndrome and its relationship with endocrine and metabolic parameters. Gynaecol Endocrinol. 2008; 24:625–630.
[60] Lin T, Li S, Xu H, Zhou H, Feng R, et al. Gastrointestinal hormone secretion in women with polycystic ovary syndrome: an observational study. Hum Reprod. 2015; 30:2639–2644.
[61] Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, et al. Population-level analysis of gut microbiome variation. Science. 2016; 352:560–564.
[62] Arusoglu G, Koksal G, Cinar N, Tapan S, Aksoy DY, et al. Basal and meal-stimulated ghrelin, PYY, CCK levels and satiety in lean women with polycystic ovary syndrome: effect of low-dose oral contraceptive. J Clin Endocrinol Metab. 2013; 98:4475–4482.
[63] Chen T, Long W, Zhang C, Liu S, Zhao L, et al. Fiber-utilizing capacity varies in prevotella- versus bacteroides-dominated gut microbiota. Sci Rep. 2017; 7:2594.
[64] Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015; 10:e0124599.
[65] Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016; 11:e0154090.
[66] Harada N, Hanaoka R, Horiuchi H, Kitakaze T, Mitani T, et al. Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci Rep. 2016; 6:23001.
[67] Choi S, Hwang YJ, Shin MJ, Yi H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J Microbiol Biotechnol. 2017; 27:2228–2236.
[68] Barrea L, Marzullo P, Muscogiuri G, di Somma C, Scacchi M, et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018; 31:291–301.
[69] Wang L, Zhou J, Gober HJ, Leung WT, Huang Z, et al. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021; 133:110958.
[70] Gu Y, Zhou G, Zhou F, Li Y, Wu Q, et al. Gut and Vaginal Microbiomes in PCOS: Implications for Women’s Health. Front Endocrinol (Lausanne). 2022; 13:808508.
[71] Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microb. 2018; 56:154–162.
[72] Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016; 535:65–74.
[73] Bang YJ, Hu Z, Li Y, Gattu S, Ruhn KA, et al. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science. 2021; 373:eabf9232.
[74] de Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell. 2018; 175:400-415.
[75] Fei F, Aa LX, Qi Q, Sun RB, Yan CX, et al. Paeoniflorin inhibits Th1 and Th17 cells in gut-associated lymphoid tissues to produce anti-arthritis activities. Inflammopharmacology. 2019; 27:1193–1203.
[76] Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol. 2019; 66:1–12.
[77] Zhang J, Sun Z, Jiang S, Bai X, Ma C, et al. Probiotic bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems. 2019; 4:e00017-19.
[78] Kumar PS. Sex and the subgingival microbiome: do female sex steroids affect periodontal bacteria? Periodontol 2000. 2013; 61:103–124.
[79] Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15:73.
[80] Klement R, Pazienza V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina (Kaunas). 2019; 55:84.
[81] Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018; 362:776–780
[82] Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, et al. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J Clin Gastroenterol. 2012; 46:468–481.
[83] Ahmadi S, Jamilian M, Karamali M, Tajabadi-Ebrahimi M, Jafari P, et al. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil (Camb). 2017; 20:254–261.
[84] He Y, Wang Q, Li X, Wang G, Zhao J, et al. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020; 11:5192–5204.
[85] Zhang F, Ma T, Cui P, Tamadon A, He S, et al. Diversity of the Gut Microbiota in Dihydrotestosterone-Induced PCOS Rats and the Pharmacologic Effects of Diane-35, Probiotics, and Berberine. Front Microbiol. 2019; 10:175.
[86] Rashad NM, El-Shal AS, Amin AI, Soliman MH. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J Funct Foods. 2017; 36:317–324.
[87] Ghanei N, Rezaei N, Amiri GA, Zayeri F, Makki G, et al. The probiotic supplementation reduced inflammation in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J Funct Foods. 2018; 42:306–311.
[88] Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob Proteins. 2019; 11:1236–1247.
[89] Li Y, Tan Y, Xia G, Shuai J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2023; 63:522–538.
[90] Gholizadeh Shamasbi S, Dehgan P, Mohammad-Alizadeh Charandabi S, Aliasgarzadeh A, Mirghafourvand M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, triple-blind, controlled, clinical trial. Eur J Nutr. 2019; 58:629–640.
[91] Rizk MG, Thackray VG. Intersection of polycystic ovary syndrome and the gut microbiome. J Endocr Soc. 2020; 5:bvaa177.
[92] Xue J, Li X, Liu P, Li K, Sha L, et al. Inulin and metformin ameliorate polycystic ovary syndrome via anti-inflammation and modulating gut microbiota in mice. Endocr J. 2019; 66:859–870.