Case Report
2015
March
Volume : 3
Issue : 1
Primary osteogenic sarcoma of breast
Jwala Srikala
Pdf Page Numbers :- 37-39
Jwala Srikala1,*
1Department of Radiology and Imaging, Krishna Institute of Medical Sciences, Minister Road, Secunderabad - 500003, Telangana, India
*Corresponding author: Dr. Jwala Srikala, MBBS, DMRD, DNB (Radio diagnosis), Chief Consultant Radiologist, Department of Radiology and Imaging, Krishna Institute of Medical Sciences, Minister Road, Secunderabad - 500003, Telangana, India. Email: srikalajwala@yahoo.com
Received 16 October 2014; Revised 20 November 2014; Accepted 27 December 2014; Published 31 December 2014
Citation: Jwala Srikala. Primary osteogenic sarcoma of breast. J Med Sci Res 2015; 3(1):37-39. DOI: http://dx.doi.org/10.17727/JMSR.2015/3-007
Copyright: © 2015 Jwala Srikala. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A 60-years-old lady presented with a firm to hard mass in right breast. She was investigated with a mammogram and ultrasound which showed a mass of indeterminate nature with extensive calcifications. An ultrasound guided core biopsy of the mass was performed which was reported as a sarcomatous mass and excision biopsy was requested. Excision biopsy concluded that the mass was an osteogenic sarcoma in the background of a phyllodes tumor. Patient underwent a definitive mastectomy two weeks later.
Keywords: Primary osteogenic sarcoma; breast cancer; phyllodes tumor
Full Text
Sarcomas of the breast are heterogeneous neoplasms derived from non-epithelial elements of the gland, and they represent less than 1% of breast cancers and less than 5% of all sarcomas [1]. When this tumour develops in the breast, it originates either from normal breast tissue de novo, or as metaplastic differentiation of a primary benign or malignant breast lesion. Secondary deposits from a primary bone sarcoma occur only rarely. Primary osteogenic breast cancer is usually considered a poor prognosis tumour, with high risk of disease recurrence and haematogeneous spread, most commonly to the lungs. In contrast to skeletal osteosarcoma affecting mainly young patients, primary osteosarcoma of the breast occurs in older patients, with a mean age at presentation around 65 [2].
Case report
A 60-years-old post-menopausal lady presented with a self-detected palpable mass in right breast. Medical history, physical examination and diagnostic tests including mammogram, ultrasound, percutaneous core biopsy, excision biopsy of the lesion, radiological imaging’s of chest, PET CT and lab tests were performed. Clinical examination showed small mass localised at 9 '0' clock position in periareolar region of right breast.
Diagnostic mammography revealed an ill-defined mass showing amorphous and coarse calcification in retroareolar region of right breast. No evidence of any axillary lymphadenopathy (Figure 1a, b, c, d). So it was diagnosed to be an indeterminate lesion of right breast - M3.
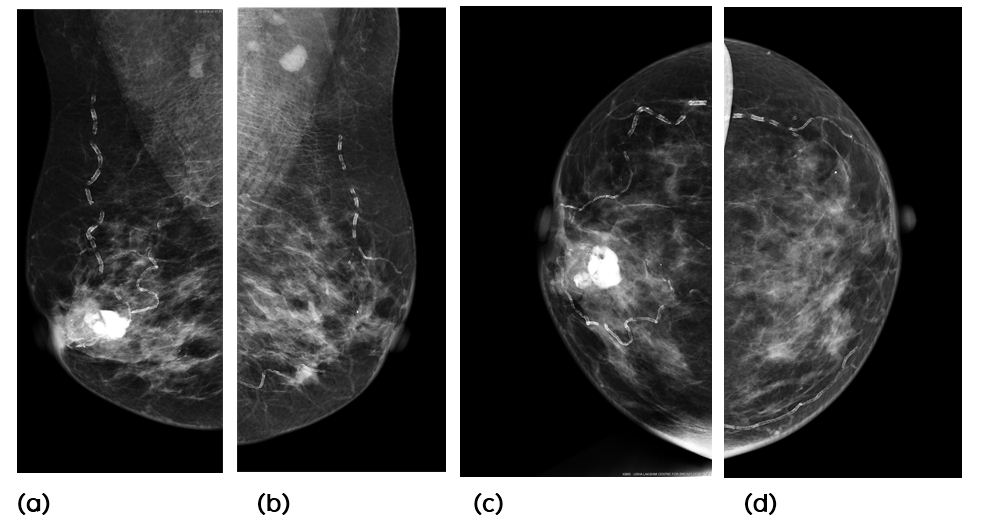
|
Figure 1a, b, c, d: Mammogram -RT and LEFT MLO, CC views: There is an ill-defined mass in the retroareolar region of right breast with amorphous and coarse calcification.
|
Sonomammogram demonstrated 2.3 x 1.9cms lobulated mass at 9 '0' clock position in periareolar region of right breast. Calcifications with posterior acoustic shadowing were noted within lesion. Few ducts around the mass were dilated. There was no evidence of axillary lymphadenopathy (Figure 2a, b, c, d).
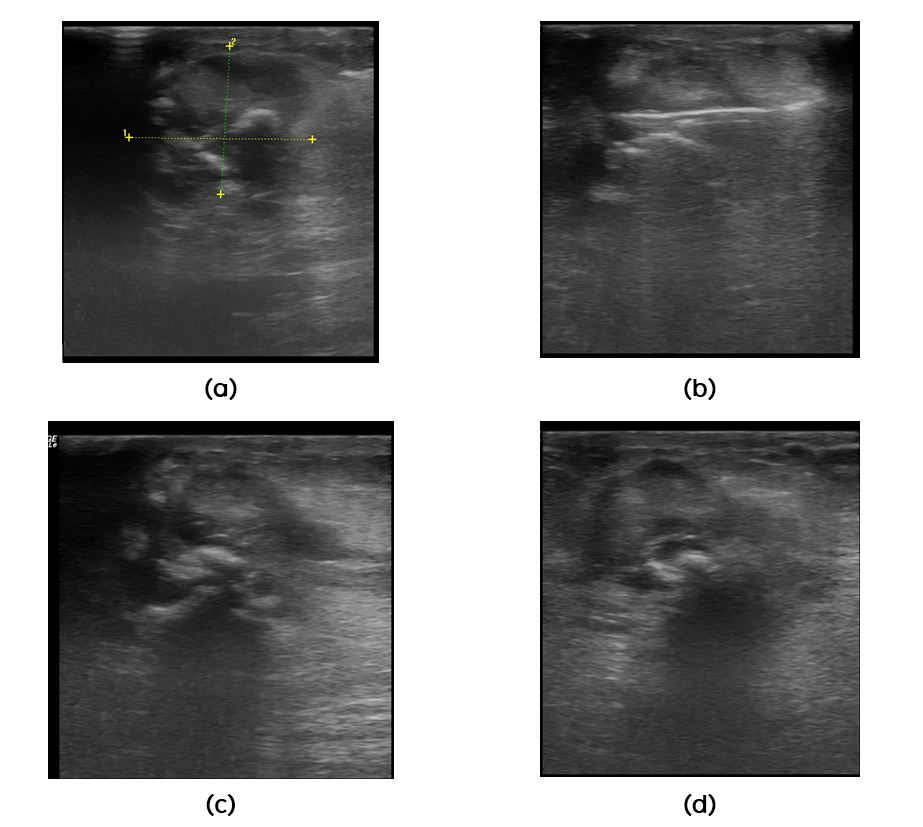
|
Figure 2a, b, c, d: Sonomammogram: There is a lobulated mass in periareolar region at 9 ‘o’ clock position with coarse calcifications.
|
Since it was a lobulated periareolar lesion of right breast with dilatation of adjacent ducts. The differential diagnosis considered were i) Papillary lesion with coarse calcifications, ii) Benign lesion with coarse calcifications, iii) Healed tuberculous mass.
Percutaneous core biopsy under ultrasound guidance was performed which showed lobules of neoplastic cartilage with matrix calcification separated by fibrocollagenous stroma. Chondrocytes were atypical. Small area of ossified bone was seen. According to the results, there was suspicion for i) Chondrosarcoma, ii) Sarcomatous component of metaplastic carcinoma. Chest radiographs and blood reports were normal.
13 days later the patient underwent surgical excision. Tumour resection with normal margins was achieved. Frozen sections revealed osteosarcoma, possible in background of phyllodes tumour. Patient underwent second stage definitive mastectomy.
PET CT was done after one week (post-operative), which revealed no radiotracer uptake elsewhere in the body except for post operative changes.
Discussion
Primary osteosarcomas of the breast are very rare and account for less than 0.1% of breast tumours [3] and are often found in women with a mean age of 64 years [4]. Extra-skeletal sarcomas occur most commonly over 50 year old age group [5], in contrast to osteogenic sarcoma arising from bone which mainly occurs in children and adolescents. The carcinogenesis of primary osteogenic sarcoma of the breast is not clear, but an origin from totipotent mesenchymal cells of the breast stroma or a transformation from a pre-existing breast lesion has been suggested. The tumour may originate from either benign breast neoplasms, such as a long standing fibroadenoma or an intraductal papilloma; or a malignant breast lesion, such as a phyllodes tumour [6].
In our case, the excision biopsy revealed osteosarcoma in the background of phyllodes tumor
Preoperative diagnosis is unusual and most patients the correct histological diagnosis of only established after surgical resection [7]. The mammographic appearances of these tumours are of a well circumscribed dense lesion within the breast tissue with focal or extensive coarse calcifications [8]. The border may be regular or irregular. The mammographic appearances may be deceptively benign and may imitate a benign fibroadenoma in a third of cases [4].
Fine needle aspiration cytology may not yield some clues to the diagnosis with features such as hypocellular or hypercellular smears with pleomorphic cells; scarce or abundant metachromatic amorphous material, suggestive of osteoid; osteoclast-like giant cells; and stromal fragments [9].
Surgical management of these tumours is either by wide local excision or mastectomy depending on the size of the tumour and remaining breast tissue. It is important to achieve a complete resection with negative resection margins, as margin status is a major factor for local disease recurrence. Axillary clearance is not necessary as these tumours do not spread via the lymphatic route. Adjuvant radiotherapy to the chest wall may reduce the risk of local recurrence [10]. The long-term prognosis is uncertain due to the small number of cases reported in the medical literature. Twenty eight percent of patients developed local recurrence and 41% distant metastases. Haematogenous metastases most commonly occur to the lungs (80%), bone (20%), and liver (17%). Prognostic factors included tumour size, number of mitoses, presence of stromal atypia, histological subtype and resection margin involvement [4].
Conclusion
Osteogenic sarcoma, although a rare tumor of the breast, should always be considered as a differential diagnosis in patients presenting with large masses with extensive coarse calcifications. Its association with phyllodes is well established in several case reports and definitive diagnosis is crucial as the surgical management of osteogenic sarcoma is different from that of the usual intraductal carcinomas of the breast.
Acknowledgements
Acknowledgements are due to the Krishna Institute of Medical Sciences (KIMS), Minister Road, Secunderabad - 500003, Telangana.
Conflict of interest
The author declares no conflict of interest.
References
1. Voutsadakis IA, Zaman K, Leyvraz S. Breast sarcomas: Current and future perspectives. The Breast. 2011; 20(3):199–204.
2. Vorobiof G, Hariparsad G, Freinkel W, Said H, Vorobiof DA. Primary osteosarcoma of the Breast: A case report. Breast J. Blackwell Science Inc; 2003; 9(3):231–233.
3. Harvey JA. Unusual breast cancers: useful clues to expanding the differential diagnosis. Radiology. United States; 2007; 242(3):683–694.
4. McGowan TS, Cummings BJ, O’Sullivan B, Catton CN, Miller N, et al. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000; 46(2):383–390.
5. Khan S, Griffiths EA, Shah N, Ravi S. Primary osteogenic sarcoma of the breast: A case report. Cases J. BioMed Central 2008 10; 1:148.
6. Ninomiya J, Oyama T, Horiguchi J, Koibuchi Y, Yoshida T, Iijima K, et al. Two cases of breast cancer with cartilaginous and osseous metaplasia. Breast Cancer. Japan; 2005;12(1):52–56.
7. Saber B, Nawal A, Mohamed F, Hassan E. Primary osteosarcoma of the breast: case report. Cases J. BioMed Central; 2008; 1:80.
8. Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998; 22(8):925–933.
9. Trihia H, Valavanis C, Markidou S, Condylis D, Poulianou E, et al. Primary osteogenic sarcoma of the breast: cytomorphologic study of 3 cases with histologic correlation. Acta Cytol. 2007; 51(3):443–450.
10. Wong L, Huang P, Luh S, Huang C. Primary leiomyosarcoma of the nipple-areola complex: Report of a case and review of literature. J Zhejiang Univ Sci B. Hangzhou: Zhejiang University Press; 2008; 9(2):109–113.