Review
2017
March
Volume : 5
Issue : 1
Principles of eye management in Stevens- Johnson syndrome
Mittanamalli S. Sridhar
Pdf Page Numbers :- 40-44
Mittanamalli S. Sridhar1,*
1Department of Ophthalmology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. M. S. Sridhar, MD., Department of Ophthalmology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Tel.: +9104044885050; Email: sri.vision@yahoo.co.in
Received 11 October 2016; Revised 12 December 2016; Accepted 20 December 2016; Published 30 December 2016
Citation: Sridhar MS. Principles of eye management in Stevens- Johnson syndrome. J Med Sci Res. 2017; 5(1):40-44. DOI: http://dx.doi.org/10.17727/JMSR.2017/5-8
Copyright: © 2017 Sridhar MS. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Stevens-Johnson syndrome and toxic epidermal necrosis though rare, are important group of diseases where an ophthalmologist plays an important role in minimizing serious eye sequel and preserving vision. Medications are common cause of this group of diseases. Infections and malignancy can also cause the same. Eye involvement in the form of conjunctivitis and denudation of eyelid margin skin are the earliest manifestations. Involving an eye surgeon whenever the diagnosis is suspected will help in early diagnosis and management. Covering the entire ocular surface and lid margin with amniotic membrane along with topical and systemic Immunosuppressive therapy are the first line-up of treatment in a diagnosed case with eye manifestations. These patients need to be followed up regularly for membrane formation, early symblepharon formation and corneal ulcers. Keratinized eyelid margin and inner eyelid surface can lead to chronic inflammation and neovascularisation of cornea. Aqueous, mucin and lipid tear deficiency can all occur. Mucous membrane grafts, scleral contact lens and autologous cultivated oral mucosal epithelial transplantations are procedures performed to stabilize the ocular surface. Keratoprosthesis is required to restore visual function.
Keywords: Stevens-Johnson syndrome; Toxic epidermal necrosis; drug reactions; amniotic membrane transplantation
Full Text
Stevens-Johnson syndrome (SJS) is an acute self-limiting disorder with immunologic dermobullous condition involving the skin and the mucous membrane of the body. SJS & Toxic epidermal necrosis (TEN) are rare blistering disorders, in which an ophthalmologist plays an important role in preventing or minimizing serious eye damage. SJS is the term used when the denuded surface involves less than 10% of the total body area. TEN is the term used when 30% of the body surface area is denuded and SJS-TEN when the detachment involves 10-30% of the body surface area [1].
Common causes of SJS are medications, infection and idiopathic. Drug related erythema multiforme manifests within 3 weeks of initiation of therapy. Severe reaction occurs within hours if re-exposure of medication occurs. Common drugs causing SJS include antibiotics like sulphonamides, phenytoin, barbiturates, penicillin and salicylates. Eye drops including scopolamine, sulphonamide and tropicamide can also cause SJS. Infections causing SJS include herpes simplex virus, mycoplasma pneumoniae and measles. Small pox vaccination and malignancy can also cause SJS.
No definitive mechanism is known for this disease. It is an autoimmune lymphocytic response triggered by drug. The primary cause is drug. Aberrant drug metabolism seems to be the cause. HLA markers have been associated. Genetic associations have been found for HLA-B12, its sub-group HLA-Bw44 and HLA-DQB1*0601 [2].
The ocular surface represents one of the major targets for SJS and TEN. Ocular involvement in acute phase of SJS/TEN occur due to rapid onset of keratinocyte apoptosis, secondary effect of inflammation and loss of ocular surface epithelium. Acute ocular involvement is reported to occur in 50% to 88% of SJS/TEN cases.
Early manifestations in eye include non-specific conjunctivitis which can precede skin eruptions. Conjunctivitis can be catarrhal/pseudomembranous or purulent. Fluoroscein staining will reveal denuded epithelium [3]. Epithelial sloughing of the ocular surface and sloughing of eyelid margin skin are early signs. Pseudomembranes, membranes, early symblepharon formation, fornix foreshortening, corneal ulceration and perforation are other common signs seen in this situation.
Acute eye manifestation of SJS/TEN can lead to chronic eye sequelae with visual significance in at least one third of patients [4]. Symblepharon and ankyloblepharon are common. This disrupts the tear film meniscus. Improper eyelid closure and restriction of ocular motility can occur. Tarsal conjunctival scaring can lead to ectropion, entropion, trichiasis, distichiasis, meibomian gland atrophy, punctal occlusion, keratinisation of eyelid margins, tarsal and bulbar conjunctival surfaces. Misdirected and or distichiatic lashes can occur. Metaplastic meibomian glands can abrade the corneal epithelium, causing epithelial defects, infection and scarring. Repeated friction from the keratinised inner eyelid surface can lead to chronic corneal inflammation, neovascularisation, scarring and limbal stem cell deficiency. Scarring in the fornices and in the lacrimal glands ducts causes severe aqueous tear deficiency and xerosis. Aqueous, mucous and lipid tear deficiency can all occur. Mucin deficiency results from loss of conjunctival goblet cells. Lipid tear deficiency results from meibomian gland inspissation and atrophy.
Corneal imaging using in-vivo confocal microscopy in chronic SJS/TEN has shown squamous epithelial metaplasia, reduced density and beading of sub-basal corneal nerves and increased number of dendritiform cells in the corneal stroma [5].
Principles of management
Every patient suspected to have acute SJS/TEN should consult ophthalmologist at the onset of the disease. Regular evaluation every 24-48 hrs is required in the acute phase.
General management includes intravenous steroids, intravenous cyclophosphamide, plasmapheresis, cyclosporine, intravenous immunoglobulins, anti-TNF agents, wound dressings, fluid management, nutrition, pain management and prevention of infection. The effect of systemic therapy for acute SJS/TEN on subsequent ocular manifestations is equivocal.
Ocular Management includes the following (a) Intravenous steroids in the acute phase is a double edged sword. It can rapidly reduce the inflammation with associated risks of gastrointestinal bleeding or sepsis. (b) Topical corticosteroid eye drops is extremely useful as anti-inflammatory therapy. Patients on steroid eye drops should be in prompt follow up. In any doubtful microbial keratitis, steroid drops need to be avoided. (c) Prophylactic antibiotics like chloramphenicol eye drops to prevent infection. (d) Covering the entire ocular surface and lid margins with amniotic membrane [6]. (e) Managing epithelial defect with preservative free artificial tears, lubricating ointment, prophylactic antibiotic drops, punctal plugs/cautery, autologous serum or plasminogen rich plasma, temporary or permanent tarsorrhaphy. (f) Mucous membrane grafts harvested from oral cavity to the lid margins and ocular surface. (g) Scleral contact lens for hydration of the ocular surface and vision improvement by reducing the irregular astigmatism of the cornea [7]. (h) Symblepharon rings and scleral contact lenses are used to prevent symblepharon and to avoid exposure keratopathy. (i) Corneal transplantation/stem cell transplantation/keratoprosthesis for vision improvement.
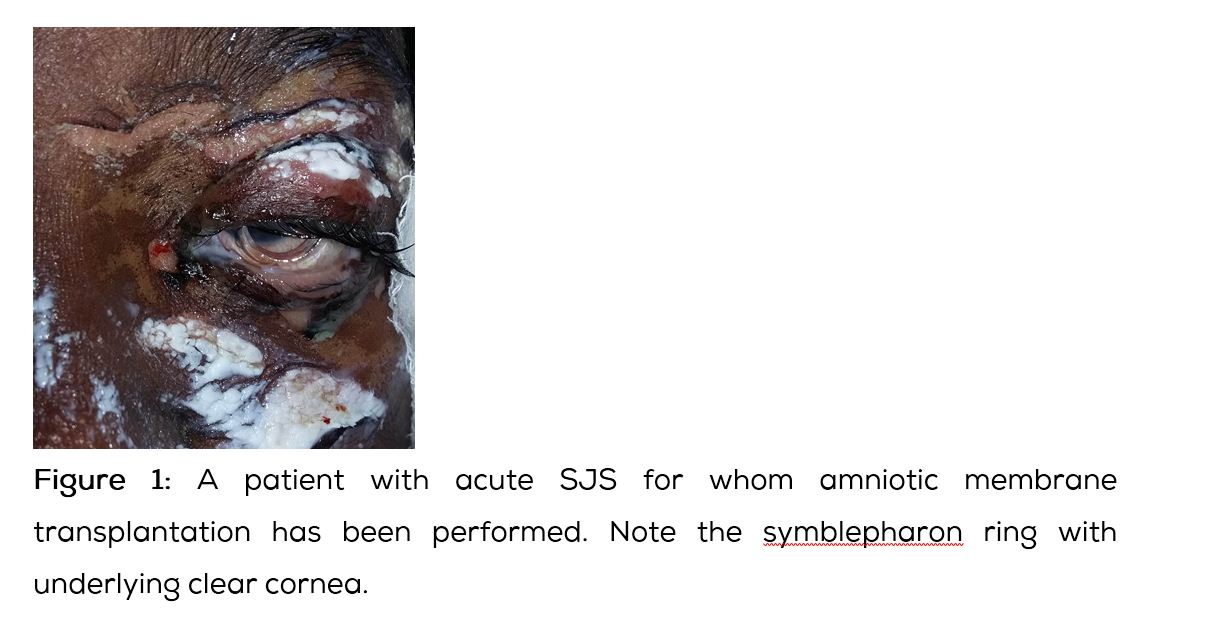
Amniotic membrane is the innermost layer of placental or fetal membrane. It has a thick basement membrane and avascular stromal matrix. It seems to have an inherent protective function. Transplantation of basement membrane leads to rapid epithelialisation, return of normal epithelial phenotype, reduced inflammation, reduced vascularisation and reduced scarring. These are the observed clinical effects of amniotic membrane transplantation. In eye, amniotic membrane transplantation is found to facilitate migration of epithelial cells, reinforces adhesion, promotes epithelial differentiation and prevents epithelial apoptosis. The epithelial cells release fibroblast growth factor, hepatocyte growth factor and transforming growth factor ß. A large piece of amniotic membrane can act as a bandage contact lens. Amniotic membrane can inhibit the influx of inflammatory cells and inhibits protease activity. The stroma inhibits invasion of new vessels. The anti- scarring effect is because of suppression of TGF ß, DNA synthesis and subsequent myofibroblast differentiation. It is an ideal substrate for supporting the growth of epithelial progenitor cells. The alpha chains of type IV collagen is identical to that in conjunctiva, but not cornea suggesting that amniotic membrane is a good conjunctival replacement.
Covering the entire ocular surface and lid margins in the acute phase of SJS/TEN has shown to improve the ocular prognosis [8]. The membrane is applied flat to cover the entire glow including the fornices and corners. The membrane is then secured to the upper and lower eyelid skin with partial thickness placement of 8-0 nylon or prolene horizontal mattress sutures with or without bolsters. Novel surgical techniques like utilizing a large sheet of amniotic membrane and a custom made forniceal ring which facilitates amniotic membrane placement has been described [9].
Management of chronic SJS 30-50% of patients with acute SJS/TEN will go on to develop chronic manifestations. These patients progress to ocular surface failure, recurrent episodic inflammation and progressive cicatricial changes.
Systemic immunosuppressive therapy has been used successfully in patients with moderate to severe ocular inflammation. Medications tried include cyclosporine, azathioprine, cyclophosphamide, methotrexate, mycophenolate and infliximab. Lagophthalmos can be corrected with release of cicatrix in the skin with or without tarsorrhaphy. Entropion and ectropion can be treated with lateral canthoplasty or tarsal strip, anterior lamellar repositioning, tarsal fracture, posterior lamellar tightening or tarsoconjunctival advancement.
Trichiasis/dischiasis recurs on epilation. Hyfrecation, cryotherapy and/or extirpation are necessary.
Dry eye syndrome is managed with preservative free artificial tears, lubricating ointment, punctal plugs and punctal cantery. Use of topical cyclosporine A may be limited by patient intolerance to the preparation. Persistent corneal epithelial defect can be managed by general principles as in acute phase.
Topical vitamin A (all trans retinoic acid ointment 0.01%) has been found useful in reducing keratinisation in patient with chronic SJS/TEN [8]. To prevent corneal damage from posterior lid margin keratinisation in SJS/TEN use of a large diameter, rigid gas permeable scleral contact lens can be final. A surgical option for correction is autologous, oral mucous membrane grafting which replaces keratinised tarsal conjunctiva with labial or buccal mucosa from the same patient [10].
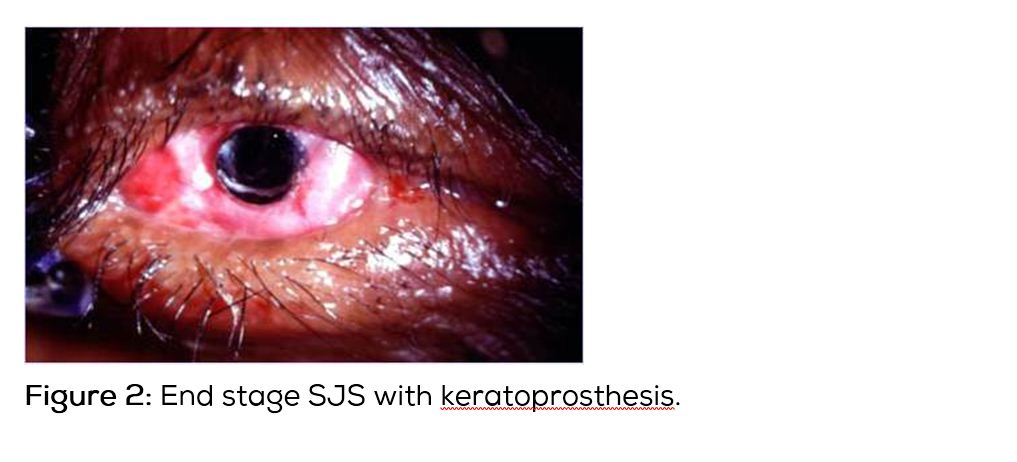
Ocular surface reconstruction includes stabilising procedures and keratoprosthesis [11, 12]. Restoration of normal eyelid/globe anatomical relationships and to the degree possible improving the tear film need to be targeted. These include punctal occlusion, mucous membrane transplantation, amniotic membrane transplantation (Figure 1) or autologous cultivated oral mucosal epithelial transplantation. For severe corneal opacity & neovascularisation limbal stem cell deficiency after SJS/TEN, keratoprosthesis (Figure 2) can restore normal or near normal visual function. Boston keratoprosthesis (type I & II) and osteo-odonto-keratoprosthesis are the procedures commonly performed [13]. Currently available keratoprosthesis, Boston type I is performed for corneal opacity with mild dry eyes. In severe dry eyes, Boston type II keratoprosthesis and modified osteo-odonto-keratoprosthesis are performed. Keratoprosthesis implantation in patients with SJS/TEN is considered as an operation of last resort because of the complications following the procedure and also the short retention time of the device. Recent advances in keratoprosthesis surgery have lowered infection rates and improved device retention. Modified osteo-odonto-keratoprosthesis appear to have longer retention rate than Boston keratoprosthesis designs. Modified osteo-odonto-keratoprosthesis procedure is time consuming and is completed in two or more stages. All patients of SJS/TEN may not be candidates for this procedure, in part because of the need of at least one viable cuspid tooth. It is extremely important to maintain good oral hygiene of all patients of SJS/TEN, so that healthy oral mucous membrane and health of the teeth is maintained for future procedures. It is better to involve a dental surgeon for this purpose. Published case series indicate the cautious use of keratoprosthesis after SJS/TEN appear to be superior to standard keratoplasty. The keratoprosthesis surgery is complex and requires intensive follow-up. It is important for highly trained surgeons to perform this procedure in referral centers and follow-up these patients regularly for better anatomical and visual outcome.
Conclusions
Understanding the various components in the pathogenesis of the Stevens-Johnson syndrome/ toxic epidermal necrosis is extremely important in preventing serious vision threatening consequences of this disease. General public should be made aware of this serious disease. General practitioners should be educated to avoid medications which cause this disease when suitable alternatives are available. In the early stages of disease, a team approach to manage hydration, nutrition, inflammation, and prevention of infection should be aggressively employed not only to save the eyes but also the life of the patient. Newer surgical procedures like amniotic membrane transplantation, mucous membrane grafting, autologous cultivated oral mucosal epithelial transplantation and keratoprosthesis are important to restore ocular surface and to improve vision. Psychological rehabilitation of the patient and family to face this traumatic event should always be included in management.
Conflicts of interest
Author declares no conflicts of interest.
References
[1] Jain R, Sharma N, Basu S, Iyer G, Ueta M, et al. Stevens–Johnson syndrome: The role of an ophthalmologist. Surv Ophthalmol. 2016; 61(4):369–399.
[2] Mondino BJ, Brown SI, Biglan AW. HLA antigens in Stevens-Johnson syndrome with ocular involvement. Arch Ophthalmol. 1982:100(9):1453–1454.
[3] Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, et al. Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology. 2009; 116(4):685–690.
[4] Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome. Toxic epidermal necrolysis - A comprehensive review and guide to therapy II. Ophthalmic disease. Ocul Surf. 2016; 14(2):168–188.
[5] Vera LS, Gueudry J, Delcampe A, Roujeau JC, Brasseur G, et al. In vivo confocal microscopic evaluation of corneal changes in chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea. 2009; 28(4):401–407.
[6] Shammas MC, Lai EC, Sarkar JS, Yang J, Starr CE, et al. Management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis utilizing amniotic membrane and topical corticosteriods. Am J Ophthalmol. 2010; 149(2):203–213.
[7] Tougeron-Brousseau B, Delcampe A, Gueudry J, Vera L, Doan S, et al. Vision-related function after scleral lens fitting in ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009; 148(6):852–859.
[8] Kobayashi A, Yoshita T, Sugiyama K, Miyashita K, Niida Y, et al. Amniotic membrane transplantation in acute phase of toxic epidermal necrolysis with severe corneal involvement. Ophthalmology. 2006; 113(1):126–132.
[9] Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf 2016; 14:31–36.
[10] Soong HK, Martin NF, Wagoner MD, Alfonso E, Mandelbaum SH, et al. Topical retinoid therapy for squamous metaplasia of various ocular surface disorders. A multicenter, placebo-controlled double-masked study. Ophthalmology. 1988; 95(10):1442–1446.
[11] Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004; 351(12):1187–1196.
[12] Iyer G, Srinivasan B, Agarwal S, Pillai VS, Ahuja A. Treatment modalities and clinical outcomes in ocular sequelae of Stevens-Johnson syndrome over 25 years - A paradigm shift. Cornea. 2016; 35(1):46–50.
[13] Tan A, Tan DT, Tan XW, Mehta JS. Osteo-odonto keratoprosthesis: Systematic review of surgical outcomes and complication rates. Ocul Surf. 2012; 10(1):15–25.