Full Text
Significant advances are being made in our approach to diagnose and manage several human diseases/disorders across the world. These advances are expected to have a significant impact on how we practice medicine in the future. It is the purpose of this column to bring these latest advances to the attention of all.
1. Nano-vehicles for Cancer Drugs
Abstract
Experimental cancer therapeutics delivered to tumors via nanoparticles could provide a safer and more effective therapy compared to conventional chemotherapy.
Introduction
Great things sometimes come in small packages. One such small packages, the smallest packages of all—nanoparticles—can deliver potent cancer drugs directly to the site of the tumor not only are able to eliminate cancer cells effectively but will also minimize side effects of chemotherapy.
Lipid-based delivery
Back in mid-1970s, it was shown that nano-sized, spherical structures form spontaneously when naturally occurring or synthetic lipids are exposed to water-now known as liposomes. Soon it was realized that liposomes could be used to carry drugs to diseased cells and tissues.
Following this, nanoparticles were developed as chains of hydrocarbons known as polymers. These targeted nanoparticle therapies can effectively deliver drug cargo to tumors, while sparing the rest of the body’s cells from the drug’s toxic effects. Indeed, both types of nanoparticles are in clinical development as cancer-drug delivery vehicles, and some liposome-based are already available for clinical use. MyoCet, for example, a liposome containing chemotherapeutic agent doxorubicin, was approved in 2004 in Canada and Europe for treatment of metastatic breast cancer. DOXIL, another lipid-enclosed doxorubicin formulation, is approved to treat AIDS-related Kaposi sarcoma; ovarian cancer (if first line treatment fails); and also multiple myeloma.
There are now a total of three nanoparticles on the market as cancer therapies, and at least a dozen more are currently making their way through clinical trials. Virtually any cancer drug can be enclosed within a nanoparticle, and they range in size from small chemotherapy drugs to larger macromolecules, such as nucleic acids. Alnylam Pharmaceuticals is currently investigating a drug candidate composed of a lipid-shell containing a small interfering RNA (siRNA) that can destroy tumors by decreasing the expression of two gene products that are involved in tumor growth: kinesin spindle protein (KSP), involved in cancer proliferation, and vascular endothelial growth factor (VEGF), involved in the growth of new blood vessels that feed tumors. Known as ALN-VSP, the drug passed through Phase 1 trials earlier this year, demonstrating anti-tumor activity in patients with liver cancer and other solid tumors that have spread to the liver.
Controlled release
The liposome platform is limited, however, in that it cannot release the drug into the tumor in a regulated way. Despite many years of research, we are yet to understand the exact mechanism of drug release from liposomes, and may involve complex processes such as disruption of the liposome membrane or fusion with cellular membranes. In fact, the actual rate of drug release from liposomes in tumors even for a marketed product like DOXIL is not completely known. As a result, there are examples where liposomal drugs have failed in clinical trials because they released the drug too slowly or too quickly. In contrast, the polymer-based nanoparticles may allow designing treatments that release the chosen drug at a predictable rate controlled by diffusion.
One such polymer-based nanoparticles that has been created for cancer treatment consists of mixing poly-lactide (PLA), a biodegradable polyester that has been used in US Food and Drug Administration-approved medical devices and pharmaceutical products for several decades, and polyethylene glycol (PEG), a biocompatible polymer commonly used in pharmaceuticals. PEG absorbs water, forming a hydration layer that encloses anti-cancer drugs and prevents the binding of blood proteins such as antibodies that might trigger the removal of the nanoparticles by the immune system. The PLA component, on the other hand, controls the release of the drug. Once in the body, the PLA layer dissolves slowly, allowing for slow diffusion release of the drug from the particle. At the same time PLA and PEG are being layered, the nanoparticle’s surface is decorated with small peptides that help the nanoparticle specifically locate and bind to the tumor’s surface. As a result, tumor cells are exposed to a higher drug concentration over a prolonged period of time than they are when treated with a conventional docetaxel regimen.
Using this PLA-PEG strategy, nanoparticles for a variety of cancers, including prostate and lung cancer are being developed. For instance, BIND-014, a drug under development, contains the FDA-approved cancer drug docetaxel and is covered in ligands that interact with a common tumor antigen known as prostate-specific membrane antigen (PSMA), recently completed a Phase 1 clinical trial involving 28 patients with advanced or metastatic solid tumors. The results, presented in April 2013 at the annual meeting of the American Association for Cancer Research (AACR), showed that BIND-014 inhibited tumor growth, and in one patient, completely resolved the disease. The data also showed that the tumors were exposed to BIND014 for a longer period of time thanks to a longer half-life than conventional docetaxel, and that a lower dose was needed to achieve a comparable level of anti-tumor activity as treatment with unpackaged docetaxel, which translated into fewer side effects for patients. This BIND-014 will commence Phase 2 trials soon.
Based on the progress made so far in the areas of nanoparticles and liposomes, it is perceived that nanotechnology will not only address simple problems such as changing the distribution of drugs to reduce toxicity or increase efficacy but it is anticipated that the future for this technology is limitless. Nanoformulations of the future will be complex and multifunctional, designed to deal with complex disease indications.
2. Watching the Brain Remember
Abstract
For the first time, one can visualize memory retrieval in real time.
Introduction
It has always been fascinating to understand how our brain stores memory and how it retrieves it when needed. Despite many years of research the way memory is stored in the brain, the molecular mechanism(s) involved in the storage and retrieval of memory remained a mystery. For the first time, scientists could catch a glimpse of memory in action.
Zebrafish as a model to study memory
The transparent brains of zebrafish, in combination with a fluorescent protein expressed in the brain in response to neural activity, have allowed researchers to catch a glimpse of memory in action.
It has been a long struggle to understand the multistep processes involved in learning and memory. Evidence that links retrieval of a specific memory to a specific set of neurons has been lacking in mammalian brains. Instead, hitherto one has to rely on more indirect experiments, such as ablating specific brain areas and testing the impact on ones’ abilities to form or retrieve memories.
In this context, Zebrafish comes handy. The transparency of zebrafish brain tissue makes the animals ideal models for studying neuronal activity. Zebrafish brains create and store memories in a remarkably similar fashion to mammalian brains, suggesting that genes important in learning and memory in the fish have mammalian homologs performing similar tasks. Another boon for memory research is the fact that during development, fish neural tubes fold outward instead of inward like mammalian brains, so that important areas buried deep in mammalian brains are located at the surface of a fish’s brain.
To study memory processes, zebrafish was trained to swim to different sides of a tank in response to different colored lights. A transgenic line of zebrafish was created whose brains express a fluorescent protein bright enough to be detected through the thin layers of tissue surrounding the fish’s cranium. The fluorescence dims in the presence of calcium, which floods neurons about a second after activation. Decreases in fluorescence, visualized using confocal microscopy, signify the brain area being utilized when the fish retrieve their memories in response to the colored lights (see Figure 1). This strategy is akin to an fMRI, except the resolution is way higher—even down to individual cells.
The zebrafish first learned that a red light heralded an electric shock, which they could escape by swimming to the other side of the tank. At 30 minutes or 24 hours after training, the zebrafish were tested for their memory retrieval capacity by immobilizing the fish with a muscle relaxant and showing them a red light. Only in trained fish given a day’s rest before testing did one can see an influx of calcium in the telencephalon, a brain region that correlates with the mammalian cortex where long-term memories are stored. The results suggest that the fish were accessing the behavioral memory of swimming away from the anticipated shock.
If the telencephalon was removed a day before the zebrafish were trained, the fish tested 24 hours after training did not remember to swim away from the light. But fish tested just 30 minutes after training still retained their aversion to red light as a short-term memory, and successfully swam away.
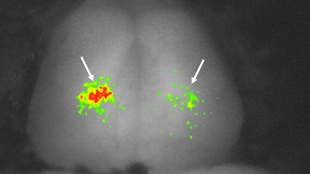
Figure 1: Active neurons in a zebrafish telencephalon.
The current technique is not sensitive enough to identify areas important for memory formation, but this technique could be upgraded to to two-photon microscopy as a result of which the fluorescent signal scatters less through tissue, allowing detection of smaller calcium changes even deeper inside the brain. This may also allow one to identify specific sets of neurons involved in guiding particular behaviors.
Reference
Aoki T, et al. Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 2013, 78: 1-14.
3. A New class of immune cells protect against type 2 diabetes mellitus
Abstract
A new class of immune cells has been discovered that protect against type 1 diabetes by suppressing other immune cells.
Introduction
Circulating T cells play a major role in protecting our body tissues against infections, tumors, and other threats. These T cells are watched by regulatory T-cells, which suppress the proliferation of other T-cells and prevent our immune systems from turning against our own bodies such as autoimmune diseases.
A new type of these suppressors that are distinguished by a protein called CD52, which they produce in large quantities and release have been discovered. These “CD52hi cells” seem to play a key in the prevention of type 1 diabetes. In humans with type 1 diabetes mellitus, these CD52hi cells are rare and ineffective at suppressing other T-cells. Furthermore, depleting these cells in diabetes-prone mice quickly triggers the disease.
The discovery of CD52hi cells could explain some puzzling side effects of alemtuzumab (or Campath)—an antibody that binds to CD52. Discovered in the 1980s, the drug has been used to successfully treat leukaemia and lymphoma—possibly because it stops cancer cells from using CD52 to suppress other T-cells and sneak past the immune system.
More recently, alemtuzumab has been used to treat multiple sclerosis, but some clinical trials have shown that 30 percent of patients who take the antibody develop a different autoimmune disease. This may be because the antibody lifts the suppressive effects of CD52hi cells, allowing pathogen-attacking immune cells to go off the rails. This may also explain why people on alemtuzumab tend to be protected against serious infections.
Subjects with type 1 diabetes often produce antibodies against GAD65. Injections of GAD65 can activate regulatory T-cells that prevent diabetes in mice. The T-cells activated by GAD65 differed from all known varieties. Their distinguishing feature was a high level of CD52, a protein with no clear physiological role that is thought to sit in the membranes of white blood cells. But the suppressive CD52hi cells can release CD52 to control other T-cells at a distance. Once it reaches other cells, it binds to the T-cell surface protein Siglec-10. This chain of events could be important for preventing type 1 diabetes. Depletion of CD52 from lymphocytes and injected these lymphocytes to young diabetes-prone mice, all of them developed diabetes within a month, far sooner than they normally would. Conversely, boosting CD52hi cells, or targeting the Siglec-10 receptor directly, could be used to treat type 1 diabetes. And conversely, blocking CD52 might be useful for boosting immune responses, and improve vaccine effectiveness.
Reference
Bandala-Sanchez E, et al. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nature Immunology, doi:10.1038/ni.2610, 2013.
4. How viruses trigger epilepsy
Abstract
Viral infections of the central nervous system may trigger cytokines that induce seizures.
Introduction
There are approximately 2.2 million Americans (in India this number could be ~ 8-9 million) affected by epilepsy, a third of which do not respond to current anti-seizure treatments, and nearly three quarters of all cases have unknown causes. Previously, more than 100 species of viruses that infect the central nervous system (CNS) have been linked to patients suffering from recurring seizures. But, still the significance of viral infections in epilepsy is unclear.
Viruses trigger generation of pro-inflammatory cytokines that could induce epilepsy
A mouse model that suffered from seizures after infection with a CNS-targeting virus has been developed to study the relationship between viral infections of CNS and epilepsy. In this model, it was observed that though the mice could clear the infection, they often continued to have seizures. In this model of post-viral epileptic seizures model, it was observed that there occurred a greater number of infiltration of the brain by macrophages (see the figure 2; green fluorescence refers to macrophages).
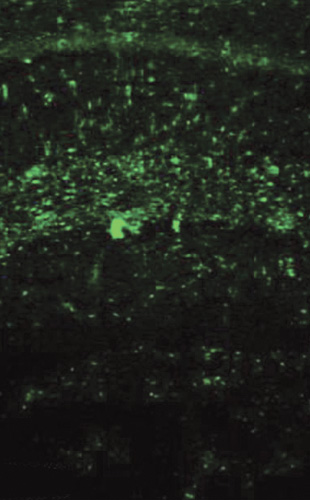
Figure 2: Green fluorescence refers to macrophages.
These infiltrating macrophages produce high levels of pro-inflammatory cytokine: interleukin-6 (IL-6), which is known to irritate the brain substance and induce seizures. Drugs that block IL-6 produced less number of seizures after the infection in this model. This suggests that macrophages continue to swarm the CNS even after the infection is cleared and produce seizures.
Reference
Cusick MF, et al. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol 2013, 87: 1849-1860.
5. Can the progress of osteoarthritis be halted
Abstract
Anti-TGFβ1 antibody may be of benefit in halting or reversing osteoarthritis. When placed in the bone (green) beneath the cartilage (red) of a rat’s knee joint, antibodies against the protein TGF-beta1 can prevent the damage caused by osteoarthritis. In Figure 3, Left, without treatment; right, with treatment. (Source: Johns Hopkins/Gehua Zhen, courtesy of Nature Medicine) Instead of seeing the painful degenerative disease as a problem primarily of the cartilage that cushions joints, recent evidence suggests that the bone underneath the cartilage is also a key player and exacerbates the damage. In a proof-of-concept experiment, it was found that blocking the action of TGF-β, a critical bone regulation protein in mice, halts progression of the disease.
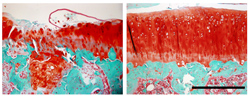
Figure 3: Left, without treatment; right, with treatment.
Introduction
The current theory on the development of osteoarthritis (OA) focuses on joint cartilage, suggesting that unstable mechanical pressure on the joints leads to more and more harm to the cartilage—and pain to the patient—until the only treatment option left is total knee or hip replacement. The new theory suggests that initial harm to the cartilage causes the bone underneath it to behave improperly by building surplus bone. The extra bone stretches the cartilage above and speeds its decline.
To prevent the grinding and wearing down of the ends of the bones at the joints, they are capped with a thin layer of cartilage, which not only provides a smooth surface for joint rotation but also absorbs some of the weight and mechanical strain placed on the joint. The degeneration of this protective layer causes extreme pain leading to limited mobility.
In osteoarthritis, degeneration is initiated by instability in the load-bearing joints of the knee and hip caused by injury or strain, so athletes, overweight people and people whose muscles are weakened by aging are at highest risk of developing OA. OA currently affects about 27 million Americans and may double by 2030. The only treatment available is pain management, or surgical replacement of the arthritic joint with a prosthetic one.
Using mice with ACL (anterior cruciate ligament) tears, which are known to lead to OA of the knee, it was found that, as soon as one week after the injury, pockets of subchondral bone had been “chewed” away by osteoclasts. This process activated high levels in the bone of TGF-beta1, which, in turn, recruited stem cells to the site so that they could create new bone to fill the holes. These pockets of new bone formation are called as “osteoid islets.” But the bone building and the bone destruction processes were not coordinated well and the bone building prevailed, placing further strain on the cartilage cap. It is this extraneous bone formation that is believed to be at the heart of OA, as confirmed in a computer simulation of the human knee.
This new finding led to the use of TGF-beta1 antibody which when was injected directly into the subchondral bone, the positive effects were seen in the bone without the negative effects on the cartilage. The same result was also seen when TGF-beta1 was genetically disrupted in the bone precursor cells alone.
These results suggest that locally applied TGF-beta1 antibodies in human patients may halt OA.
Conflicts of interest
Author declares no conflicts of interest.