Full Text
Introduction
Diabetes mellitus is a systemic chronic disease of the metabolism that progresses with chronic hyperglycemia and is characterized by disturbances in carbohydrate, protein and lipid metabolisms that appear due to a partial or complete deficiency of insulin and/or insulin resistance [1]. It is an important health problem with a prevalence that has been increasing throughout the world due to reasons such as a sedentary lifestyle, unhealthy diet and being overweight. Uncontrolled hyperglycaemia leads to microvascular complications of diabetes such as retinopathy, nephropathy, neuropathy. Diabetic nephropathy (DN) is a complication of diabetes mellitus with worst outcomes and with significant morbidity and mortality [2]. Nearly one of every three diabetic subjects develops DN during the course of their disease [3]. The American Diabetes Association (ADA), and the National Kidney Foundation (NKF) came to an agreement in 2014 and defined DN as the chronic kidney disease brought on by T2DM and characterized by a urinary albumin/creatinine ratio (ACR) of greater than 30 mg/g for more than >3 months or a persistent estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2 [4]. The 24-hour albuminuria excretion rate (AER) and glomerular filtration rate (GFR) were combined as the new categorization of diabetic nephropathy by the Joint Committee on Diabetic Nephropathy [5] and AER and spot ACR are regarded as the gold standard DN diagnostic assays [6].A decline in eGFR among T2DM patients coexists with the advancement of DN. eGFR and albuminuria has traditionally been regarded as complementary symptoms of DN [7]. However, according to studies those with T2DM who have normoalbuminuria could have serious renal problems [8, 9]. The Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) study demonstrated that 24% of the examined DM patients with baseline eGFR level > 60 ml per min per 1.73 m2 progressed to DN within one year however, they did not experience microalbuminuria or macroalbuminuria [10]. As DN is the leading cause of chronic kidney disease and failure early detection of nephropathy among T2DM is crucial so that prompt intervention can be done. Recently serum cystatin C has been considered a new biomarker for the diagnosis of renal damage. Several studies have shown that serum cystatin C is a better marker of decreased eGFR than Serum creatinine [11-15]. Cystatin C is a superior biomarker for early kidney illness, according to numerous studies. Nevertheless there are still some contradictory studies that claim cystatin C is not more sensitive than other markers in identifying early renal impairment in diabetics, children, the general population and previous kidney donors [16–20].
Some studies claim that serum cystatin is not better indicator than creatinine for identifying, nephropathy in T2DM patients [21]. Due to varying reviews on the usefulness of serum cystatin C as a biological marker for detection of renal disease prompted us to conduct a meta-analysis to systematically analyze the relevant studies for determining the predictive value of serum cystatin C for the early detection of DN in T2DM patients. In this study we aimed to evaluate the GFR via serum cystatin C for the early detection of DN among T2DM patients.
Search strategy
A computerized literature search was conducted using from September 2021 to May 2022 MeSH (medical subject headings) such as ‘serum cystatin C’ and ‘diabetic nephropathy’ and ‘glomerular filtration rate’ AND (sensitivity OR specificity OR accuracy) in PubMed, Google Scholar, Web of Science, EMBASE, and Scopus Database to identify potentially relevant articles. The titles and abstracts of each resulting studies were carefully screened. Full texts of related studies were independently evaluated for additional eligible studies. Reference lists of the identified articles were also reviewed manually to identify additional articles.
Study selection
The following inclusion criteria were used: (i) Studies involving serum cystatin C predicting DN and giving the measured glomerular filtration rate (eGFR) for patients with T2DM, (ii) Studies that include parametric numerical data of serum cystatin C (mean ± standard deviation), (iii) Studies that included humans as participants, (iv) The age of the subjects was limited (>60 yrs age), (v) Studies with individuals who had a diabetic ketoacidosis, primary kidney disease, heart failure, renal injury brought on by drugs, serious infection, cancer were not included, (vi) Studies without patient eGFR (sensitivity, specificity, or accuracy) were also disregarded.
Figure 1: Summary of the literature search.
Data extraction
Data was independently extracted for type of DM, measured values for serum cystatin C, cut-off value for serum cystatin C, specificity, sensitivity, and the Area under the curve (AUC) and Receiver operating characteristic curve (ROC) were all recorded or recalculated.
Statistical analysis
Comprehensive Meta-Analysis Software was utilized for the statistical analysis. The area under the summary receiver operating characteristic curve (SROC) was used to express the diagnostic precision of serum cystatin C for diagnosing DN.
Search results and study characteristics
A guideline for PRISMA (Reporting Items for Systematic Reviews and Meta-Analyses) was used [22]. 190 articles were found at the beginning of the cursory search. After reviewing the titles and abstracts of these, 178 articles were eliminated because they were duplicates, review articles, animal research or weren't relevant to the current analysis. We found 49 more articles that qualified for our meta-analysis by reading the potentially pertinent ones that we discovered by looking through the reference lists of the related articles and reviews. Out which only 3 were eligible for our studies. We eventually included nine studies totaling 1428 patients in the meta-analysis following a thorough and meticulous screening process (Figure 1). Tables 1 & 2 provide a list of the features of the included studies. The majority of research was carried out on T2DM patients. Turbidimetric immunoassay and nephelometric immunoassay method were used to test serum cystatin C level [23-27]. eGFR was obtained by laboratory testing, the plasma creatinine clearance or 51Cr-EDTA clearance [28, 29].
Table 1: Characteristics of the 9 studies include in the meta-analysis.
|
First author
|
Ethnicity
|
Country
|
|
Iliadis et al [26]
|
Western
|
Greece
|
|
Macsiaac et al [9]
|
Western
|
Austin
|
|
Beauvieux et al [24]
|
Western
|
France
|
|
Chae et al [27]
|
Asian
|
Korea
|
|
Bicik et al [12]
|
Eastern
|
Turkey
|
|
Rigalleau et al [31]
|
Western
|
France
|
|
Chritensson et al [23]
|
Western
|
Sweden
|
|
Bevc et al [29]
|
Western
|
Slovenia
|
|
Oddoze et al [18]
|
Western
|
France
|
Table 2: Characteristics of the 9 studies include in the meta-analysis with true positive, false positive, false negative, false positive.
|
Study
|
Sample
size
|
DN caused
by type DM
|
True
positive
|
False
positive
|
False
negative
|
False
positive
|
Serum cystatin C
(mg/dl)
threshold
|
|
Iliadis et al [26]
|
445
|
T2DM
|
118
|
60
|
27
|
241
|
1.11
|
|
Beauvieux et al [24]
|
252
|
T2DM
|
54
|
20
|
5
|
174
|
1.1
|
|
Chae et al [27]
|
122
|
T1DM & T2DM
|
71
|
5
|
6
|
43
|
0.96
|
|
Bicik et al [12]
|
113
|
T2DM
|
18
|
30
|
5
|
60
|
Not given
|
|
Rigalleau et al [31]
|
75
|
T2DM
|
30
|
4
|
3
|
35
|
1.39
|
|
Bevc et al [29]
|
126
|
T1DM & T2DM
|
13
|
5
|
1
|
102
|
1.1
|
|
Oddoze et al [18]
|
114
|
T2DM
|
73
|
2
|
13
|
27
|
1.07
|
|
Iliadis et al [26]
|
53
|
T2DM
|
9
|
4
|
1
|
35
|
0.95
|
Meta-analysis Heterogeneity analysis – Sensitivity of heterogeneity was (I2 = 58.1%, p = 0.0228). Specificity heterogeneity was (I2 = 86.3% p = 0.0010). The heterogeneity of the negative likelihood ratio was (I2 = 50.79% p = 0.0413) and heterogeneity of the positive likelihood ratio was (I2 = 86.2% p = 0.0001). The diagnostic odds ratio of heterogeneity was (I2 = 69.6% p = 0.0005).
Figures 2 to 7 display forest plots. Sensitivity ranged from 0.83 to 0.99 and the pooled sensitivity of serum cystatin C was 0.87 (95% CI 0.84 - 0.91). The specificity ranged from 0.69 to 1.02 and the pooled specificity of serum cystatin C 0.84 (95% CI 0.83 - 0.88) for predicting DN. The pooled positive predictive values of serum cystatin C was 7.035 (95% CI 4.30 – 11.43) and negative predictive values of serum cystatin C was 0.15 (95% CI 0.09 - 0.21) for predicting DN. The diagnostic odds ratio was 67.10 (95% CI 28.12 - 156.84). SROC for 9 studies AUROC= 0.9548 Q-test = 0.8971 SE (Q) = 0.0225 (Figure 7).
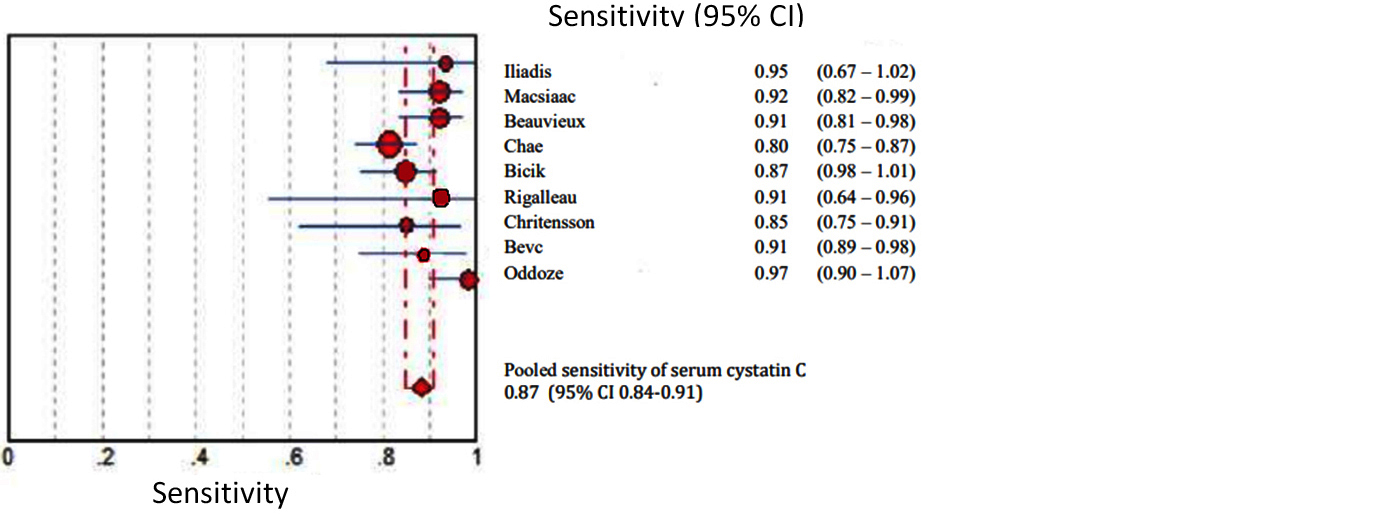
Figure 2: Shows pooled Forest plot for sensitivity of serum cystatin C for the estimation of glomerular filtration rate in predicting DN in T2DM. The diamond represents the pooled estimate with 95% CI. Horizontal lines represent 95% CI & red circles the study-specific index of diagnosis.
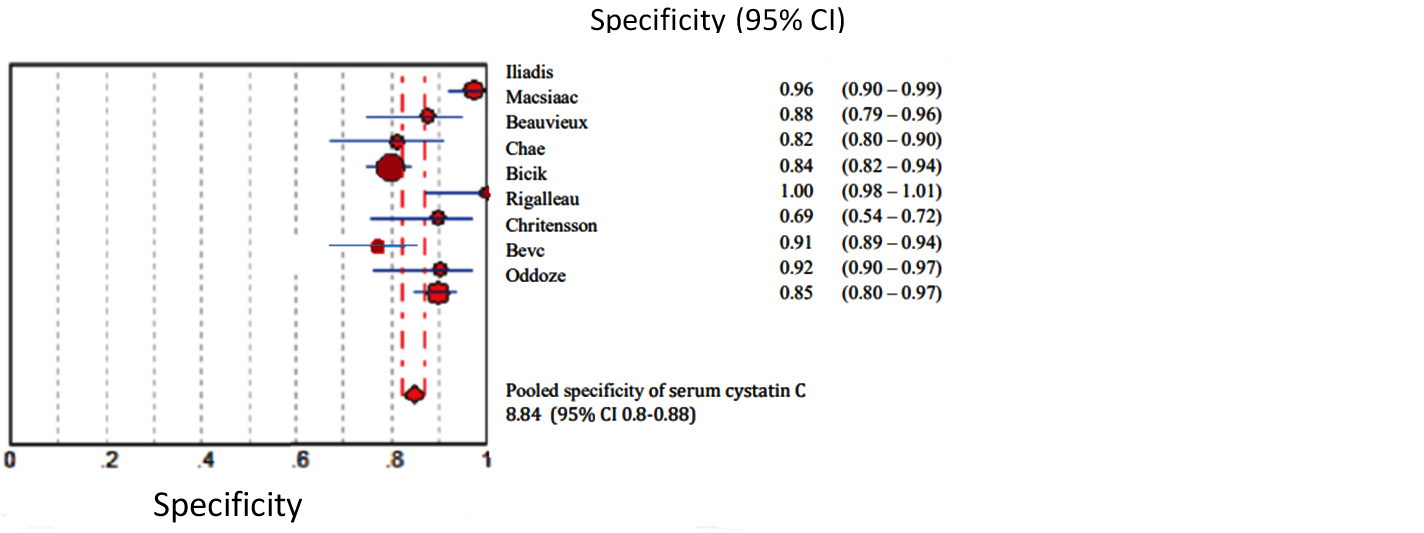
Figure 3: Shows pooled Forest plot for specificity of serum cystatin C for the estimation of glomerular filtration rate in predicting DN in T2DM. The diamond represents the pooled estimate with 95% CI. Horizontal lines represent 95% CI & red circles the study-specific index of diagnosis.
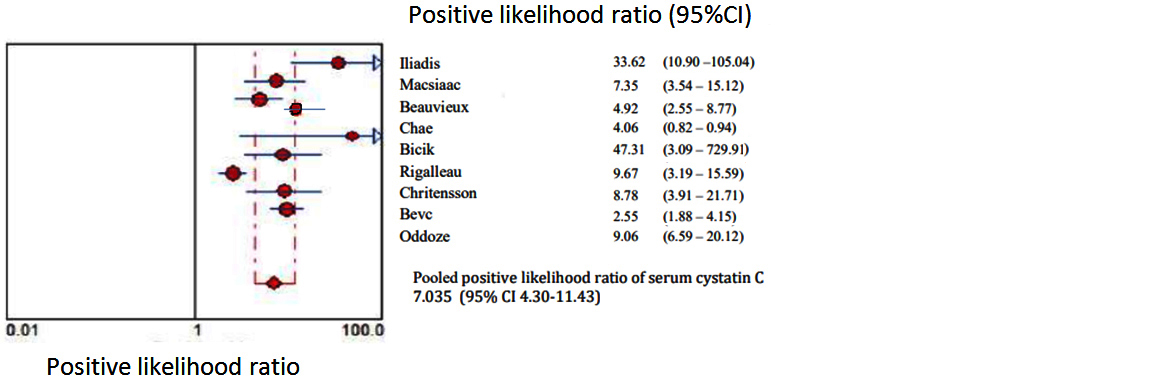
Figure 4: Shows pooled Forest plot for positive likelihood ratio of serum cystatin C for the estimation of glomerular filtration rate in predicting DN in T2DM. The diamond represents the pooled estimate with 95% CI. Horizontal lines represent 95% CI & red circles the study-specific index of diagnosis.
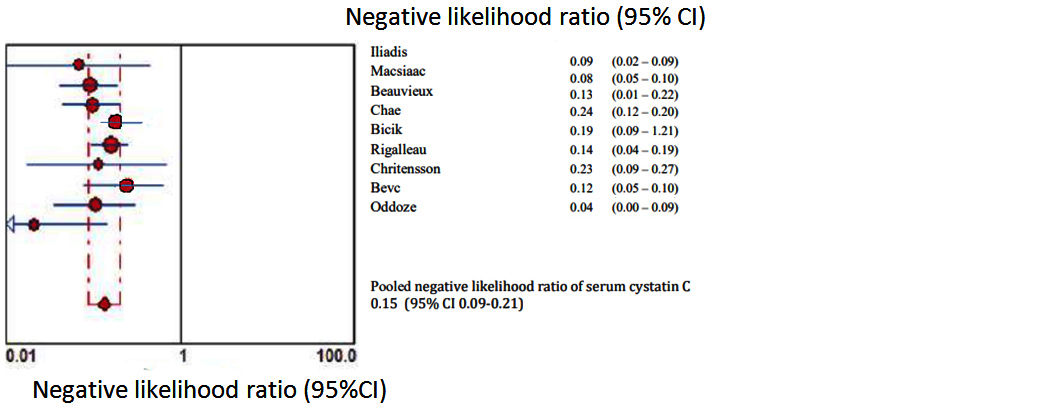
Figure 5: Shows pooled Forest plot for negative likelihood ratio of serum cystatin C for the estimation of glomerular filtration rate in predicting DN in T2DM. The diamond represents the pooled estimate with 95% CI. Horizontal lines represent 95% CI & red circles the study-specific index of diagnosis.
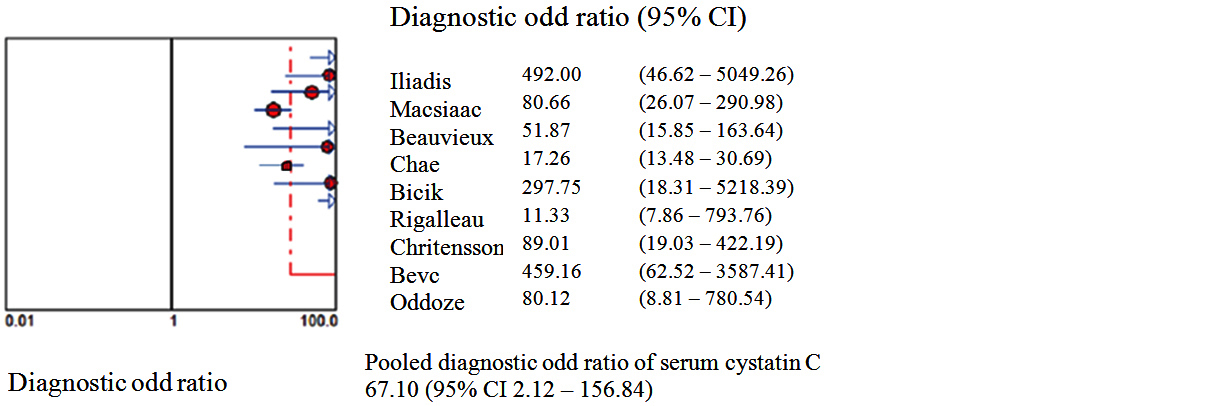
Figure 6: Shows pooled Forest plots for Diagnostic Odd Ratio of serum cystatin C for the estimation of glomerular filtration rate in predicting DN in T2DM. The diamond represents the pooled estimate with 95% CI. Horizontal lines represent 95% CI & red circles the study-specific index of diagnosis.
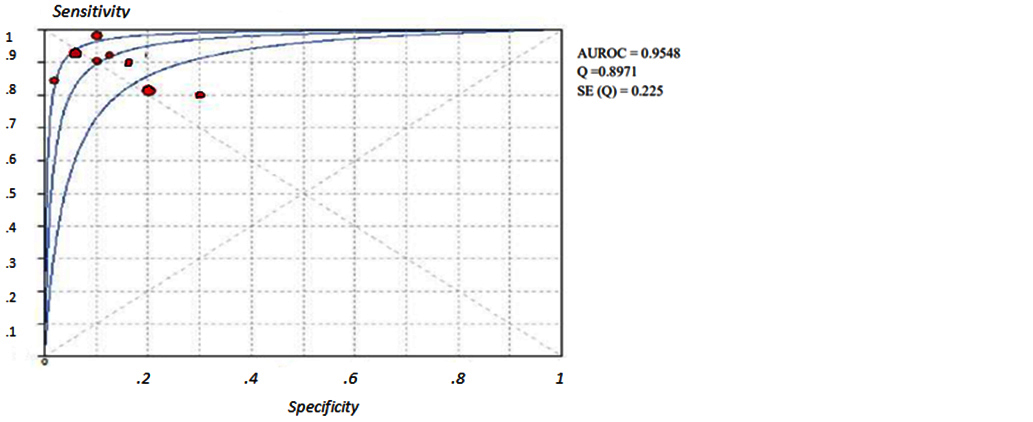
Figure 7: Shows SROC curves of serum cystatin C for the estimation of eGFR in predicting DN in T2DM.
Discussion
A third of diabetic individuals experience DN and since the overall number of diabetics is anticipated to raise the prevalence of DN will likewise sharply increase [30]. Finding a marker to accurately diagnose DN in its early phase, halt its progression to end-stage renal disease and consequently lessen the burden of patients. Serum cystatin C is a non-glycosylated low molecular weight basic protein which is produced in the body. It can be entirely eliminated from circulation through glomerular filtration followed by proximal tubular reabsorption and finally degradation [11, 31]. Serum cystatin C is often higher during the first year of life when the kidneys are still developing but the values have a tendency to rapidly fall as the kidneys get older. Normal range set 0.51-1.31 mg/dL up until the age of 50 with a constant production rate [32]. Patients with T2DM decline in eGFR is seen along with the advancement of DN. There is an increased risk of death as the patient's eGFR drops below 60 ml/min/1.73 m2 [33].Stevens et al. addressed concerns about the usage of serum cystatin C and came to the conclusion that while the factors like age, sex, and race have an impact on serum cystatin C level their influence on creatinine & eGFR is greater than that on serum cystatin C [34]. In order to predict DN the current meta-analysis compared serum cystatin C with eGFR. Nine studies including the serum cystatin C test level for the early diagnosis of DN were included in the current meta-analysis. According to the findings serum cystatin C could identify DN with pooled sensitivity of 0.87 and pooled specificity of 0.84. The chances of a disease diagnosis increased with a higher positive likelihood ratio & the likelihood of developing a given condition decreased with a smaller negative likelihood ratio. According to the pooled positive likelihood ratio of 7.055 hence T2DM patients with elevated serum cystatin C had a higher risk of developing DN. Patients with normal serum cystatin C levels had fewer instances of DN as evidenced by the pooled negative probability ratio of 0.15. SROC curve served as a general assessment of the efficacy of the diagnostic procedures. SROC area under the curve was 0.95498 and the Q value was 0.8971. Diagnostic odds ratio (67.10) is the ratio of the true positives to false positives compared to the ratio of the true negatives to false negatives. The meta-analysis revealed that the serum cystatin C level is a possible indicator of the development of DN in people with T2DM and that the values rise as the condition worsens. This finding supports earlier research and provides more proof that the serum cystatin can be used as an early indicator in clinical settings and can distinguish between different levels of kidney impairment [35].
Limitations
The individuals' varied ethnic backgrounds, the absence of non-diabetes renal disease controls and the inability to identify additional factors that could affect cystatin C levels and large-scale studies needed.
Conclusion
In this meta-analysis we concluded that serum cystatin C is a significant biomarker of DN among patients with T2DM. Serum cystatin C levels are significantly closer to accurate measurements of eGFR and are significantly more sensitive to detect early GFR impairment. It can also positively monitor kidney function progression and prediction of adverse outcomes among T2DM patients as the approach to treating diabetic nephropathy depends on stage of nephropathy. In day to day clinical practice serum cystatin C is not routinely done test as it is expensive and not widely available assay though being one of the earliest markers of DN. According to our meta-analysis we further suggest that serum cystatin C could be used in the future for grading or staging CKD in DN in T2DM patients because serum cystatin C levels rise with the stage of renal dysfunction. It may also be examined as a possible predictor of cardiovascular events in T2DM individuals with DN.
Conflict of interest
The authors declare no conflict of interest.
References
[1] Onalan E. The relationship between monocyte to high-density lipoprotein cholesterol ratio and diabetic nephropathy. Pak J Med Sci. 2019; 35(4):1081–1086.
[2] Bilgin S, Kurtkulagi O, Tel BMA, Duman TT, Kahveci G, et al., Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes. 2021; 15(6):1071–1074.
[3] Feng X, Zhao J, Ding J, Shen X, Zhou J, et al. LncRNA Blnc1 expression and its effect on renal fibrosis in diabetic nephropathy, Am J Transl Res. 2019; 11(9):5664–5672.
[4] Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, et al, Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014; 37(10):2864–2883.
[5] Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, et al. Joint committee on diabetic nephropathy: A new classification of diabetic nephropathy 2014: a report from joint committee on diabetic nephropathy. J Diabetes Investig. 2015; 6(2):242–246.
[6] Kowalski A, Krikorian A, Lerma EV. Diabetes and chronic kidney disease. Dis Mon. 2015; 61(9):378–386.
[7] De Boer IH, Steffes MW. Glomerular filtration rate and albuminuria: twin manifestations of nephropathy indiabetes. J Am Soc Nephro. 2007; 18(4):1036–1037.
[8] Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003; 52(4):1036–1040.
[9] MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004; 27(1):195–200.
[10] Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, et al. Epidemiologyof diabetes interventions and complications study group: Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010; 33(7):1536–1543.
[11] Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995; 47(1):312–318.
[12] Bevc S, Hojs R, Ekar R, Gorenjak M, Puklavec L. Simple cystatin C formula compared to serum creatinine based formula for glomerular filtration rate in patients with mildly to moderately impaired kidney function. Kidney Blood Press Res. 2012; 35(6):649–654.
[13] Mussap M, Vestra MD, Fioretto P, Saller A, Varagnolo M, et al. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002; 61(4):1453–1461.
[14] Perlemoine C, Beauvieux MC, Rigalleau V Baillet L, Barthes N, et al. Interest of cystatin C in screening diabetic patients for early impairment of renal function. Metabolism. 2003; 52(10):1258–1264.
[15] Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, et al. Cystatin C and estimates of renal function: searching for a better measure of kidney function in diabetic patients. Clin Chem. 2007; 53(3):480–488.
[16] Zaffanello M, Franchini M, Fanos V. Review: Is serum cystatin-C a suitable marker of renal function in children? Ann Clin Lab Sci. 2007; 37(3):233–240.
[17] Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010; 78(12):1305–1311.
[18] Oddoze C, Morange S, Portugal H, Berland Y, Dussol B. Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis. 2008; 38(2):310–316.
[19] Noortgate NJ, Van Den, Janssens WH, Delanghe JR. Serum cystatin C concentration compared with other markers of glomerular filtration rate. J Am Geriatr Soc. 2002; 50(7):1278–1282.
[20] Louvar DW, Rogers TB, Bailey RF, Matas AJ, Ibrahim HN. Cystatin C is not superior to creatinine-based models in estimating glomerular filtration rate in former kidney donors. Transplantation. 2007; 84(9):1112–1117.
[21] Safdar OY. Serum cystatin C is a useful biomarker but not superior to serum creatinine assessment for the diagnosis of acute kidney injury in septic children: A prospective cohort study. J Transl Sci. 2016; 2:74–78.
[22] Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 Statement. Sys Rev. 2015; 4(1):1.
[23] Christensson AG, Grubb AO, Nilsson JA, Norrgren K, Sterner G, et al. Serum cystatin C advantageous compared with serum creatinine in the detection of mild but not severe diabetic nephropathy. J Intern Med. 2004; 256(6):510–518.
[24] Beauvieux MC, Le Moigne F, Lasseur C, Raffaitin C, Perlemoine C, et al. New predictive equations improve monitoring of kidney function in patients with diabetes. Diabetes Care. 2007; 30(8):1988–1994.
[25] Rigalleau V, Beauvieux MC, Le Moigne F, Lasseur C, Chauveau P, et al. Cystatin C improves the diagnosis and stratification of chronic kidney disease, and the estimationof glomerular filtration rate in diabetes. Diabetes Metab. 2008; 34(5):482–489.
[26] Iliadis F, Didangelos T, Ntemka A, Makedou A, Moralidis E, et al. Glomerular filtration rate estimation in patients with type 2 diabetes: creatinine or cystatin C-based equations? Diabetologia. 2011; 54(12):2987–2994.
[27] Chae HW, Shin JI, Kwon AR, Kim HS, Kim DH. Spot urine albumin to creatinine ratio and serum cystatinC are effective for detection of diabetic nephropathy in childhood diabetic patients. J Korean Med Sci. 2012; 27(7):784–787.
[28] Bicik Z, Bahcebasi T, Kulaksizoglu S, Yavuz O. The efficacy of cystatin C assay in the prediction of glomerular filtration rate. Is it a more reliable marker for renal failure? Clin Chem Lab Med. 2005; 43(8):855–861.
[29] Bevc S, Hojs R, Ekart R, Završnik M, Gorenjak M, et al. Simple cystatin C formula for estimation of glomerular filtration rate in overweight patients with diabetes mellitus type 2 and chronic kidney disease. Exp Diabetes Res. 2012; 2012:179849.
[30] Reutens AT, Atkins RC. Epidemiology of diabetic nephropathy. Contrib Nephrol. 2011; 170:1–7.
[31] Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, et al. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998; 101(5):875–881.
[32] Langlois V. Laboratory evaluation at different ages. Compr Pediatr Nephrol. First Edit, Elsevier Inc. 2008; pp.39–54.
[33] Ekart R, Bevc S, Hojs N, Knehtl M, Dvoršak B, et al. Albuminuria is associated with subendocardial viability ratio in chronic kidney disease patients. Kidney Blood Press Res. 2015; 40(6):565–574.
[34] Stevens LA, Schmid CH, Greene T, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009; 75(6):652–660. [35] Zhou B, Zou H, Xu G. Clinical utility of serum cystatin C in predicting diabetic nephropathy among patients with diabetes mellitus: A meta-analysis. Kidney Blood Press Res. 2016; 41(6):919–928.