Full Text
Introduction
Leprosy, also known as Hansen’s disease, is a chronic infectious disease caused by Mycobacterium leprae, a microorganism that has a predilection for the skin and nerves [1]. In India and in most endemic countries, for purposes of therapy leprosy patients are classified into paucibacillary and multibacillary types on the basis of the number of skin lesions, with five lesions being the determining number [2]. It is one of the leading causes of physical disabilities which contributes to intense social stigma resulting in discrimination of patients and their families [3]. India has achieved the global leprosy elimination target in December 2005, and the average prevalence of the disease at the national level was 0.68/10,000 in 2015 [4]. Clinical classification gives recognition only to gross appearances of the lesions, while the parameters used for the histopathological classification are well defined, precise and also take into account the immunological manifestations which enable it to successfully bridge the pitfalls in leprosy diagnosis. Histopathology provides confirmatory information for suspected cases which can be missed in clinical practice or epidemiological studies and helps in exact typing. Histology also gives indication of progression and regression of disease under treatment [5]. In the present study emphasis is given on the clinical and histopathology of paucibacillary leprosy.
Materials and methods
The present study was undertaken in the Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, over a period of 3 years 6 months from January 2013 to June 2016. The study consisted of skin biopsies sent from the patients with clinical history suggestive of leprosy.
Methodology: Histopathological study of skin biopsy specimens from 130 clinically diagnosed and clinically suspected cases of paucibacillary leprosy. Cases of relapse and lepra reactions were also considered based on the number of lesions. A brief clinical history, examination findings indicating signs and symptoms of the skin lesions and provisional clinical diagnosis were collected.
Inclusion criteria: (i) clinically diagnosed and clinically suspected new cases of paucibacillary leprosy, (ii) Based on the number of lesions (<5), Not clinically classified cases, relapse cases and lepra reactions also included in the study.
Exclusion criteria: patients who were not clinically diagnosed as leprosy.
The skin punch biopsies measuring 0.5cmx0.5cm from the representative lesion were taken by the Dermatologists, and dispatched in glass or plastic vials containing 10% formalin solution. Following fixation for 12-24 hours the tissues were processed, embedded in paraffin and serial sections of 4-5 microns were obtained, which were stained with hematoxylin and eosin for morphological assessment and with Fite-Faraco stain for identification of the bacilli. The procedure followed for Fite-Faraco stain was the Modified Wade-Fite method.
Results
130 cases were considered for the study. Patient’s age ranged from 4 years to 80 years. 48(36.9%) patients were in the age group 16-30 years, followed by 34(26.1%), 23(17.6%), 13(10%), 10(7.6%) and 2(1.5%) were in the age group 31-45yrs, 0-15yrs, 46-60yrs, 61-75yrs and 76-90yrs respectively (Table 1).
Table 1: Demographic profile of leprosy patients.
|
Characteristic
|
Subgroups
|
No of cases(%)
Total - 130
|
|
Age
|
|
|
0-15yrs
|
23(17.6%)
|
|
|
16-30yrs
|
48(36.9%)
|
|
|
31-45yrs
|
34(26.1%)
|
|
|
46-60yrs
|
13(10%)
|
|
|
61-75yrs
|
10(7.6%)
|
|
|
76-90yrs
|
2(1.5%)
|
|
Gender
|
|
|
Male
|
81(62.3%)
|
|
|
Female
|
49(37.6%)
|
There were 81(62.3%) male and 49(37.6%) female patients, with male to female ratio of 1.6:1 (Table 1).
Table 2 shows the clinical profile of paucibacillary leprosy patients. Majority of the patients had hypopigmented patch, nerve thickening and combined lesions.
Table 2: Clinical profile of leprosy patients.
|
Characteristic symptoms
|
No of cases(%)
|
|
Hypopigmented patch
|
103(79.2%)
|
|
Erythematous patch
|
28(21.5%)
|
|
Trophic ulcers
|
6(4.6%)
|
|
Combined lesions
|
39(30%)
|
|
Nerve thickening
|
53(40.7%)
|
|
deformities
|
3(2.3%)
|
Table 3 shows the distribution of paucibacillary leprosy cases based on clinical diagnoses made and number of lesions. Out of which 63 borderline tuberculoid leprosy, 7 tuberculoid leprosy and 4 indeterminate leprosy, which comes under the clinical classification of paucibacillary leprosy. Others clinical diagnoses included 34 not clinically classified cases, 20 relapses and one case each of borderline and Type 2 reaction were also considered for the study based on the number of lesions.
Table 3: Clinical classification of paucibacillary leprosy.
|
Type of leprosy
|
No of cases
|
|
Tuberculoid leprosy
|
07
|
|
Borderline tuberculoid leprosy
|
63
|
|
Indeterminate leprosy
|
04
|
|
Others
|
|
Not clinically classified cases
|
34
|
|
Relapses
|
20
|
|
Borderline borderline
|
01
|
|
Type 2
|
01
|
Table 4 shows the distribution of paucibacillary leprosy cases on histopathology. Majority of the cases turned out to be indeterminate leprosy. In 20 cases there was no evidence of leprosy.
Table 4: Histopathological changes observed in epidermis and dermis in leprosy.
|
Changes
|
Histopathology diagnosis (No of cases)
|
|
BT (40)
|
TT (19)
|
IL (51)
|
NE (20)
|
|
Epidermis
|
|
UR
|
22
|
6
|
24
|
14
|
|
Atrophy
|
18
|
13
|
27
|
6
|
|
Dermis
|
|
Grenz zone
|
21
|
-
|
49
|
20
|
|
Granuloma
|
13
|
19
|
-
|
-
|
|
Giant Cell
|
3
|
13
|
-
|
-
|
|
Lymphocyte NV
|
39
|
17
|
48
|
-
|
|
Lymphocyte AD
|
38
|
17
|
36
|
-
|
|
Lymphocyte AP
|
11
|
11
|
8
|
-
|
|
Macrophage NV
|
30
|
9
|
17
|
-
|
|
Macrophage AD
|
29
|
6
|
10
|
-
|
|
Macrophage AP
|
9
|
2
|
2
|
-
|
|
FF stain positive
|
3
|
-
|
-
|
-
|
Abbreviations: BT – Borderline tuberculoid, TT – Tuberculoid, UR - Unremarkable, NE –No evidence of leprosy, NV – Neurovascular bundle, AD- Adnexa, AP – Arrector pili, FF – Fite Faraco.
Histopathological findings observed were as follows
Borderline tuberculoid leprosy: The findings noted were atrophy in epidermis, dermis showed grenz zone, granulomas which had giant cells in few cases, lymphocyte infiltration around neurovascular bundle and adnexal structures, macrophage infiltration around neurovascular bundle, adnexal structures and arrector pilae noted. Fite Faraco stain was positive in 3 cases among 40 cases of borderline tuberculoid leprosy (Figure 1, Table 4).
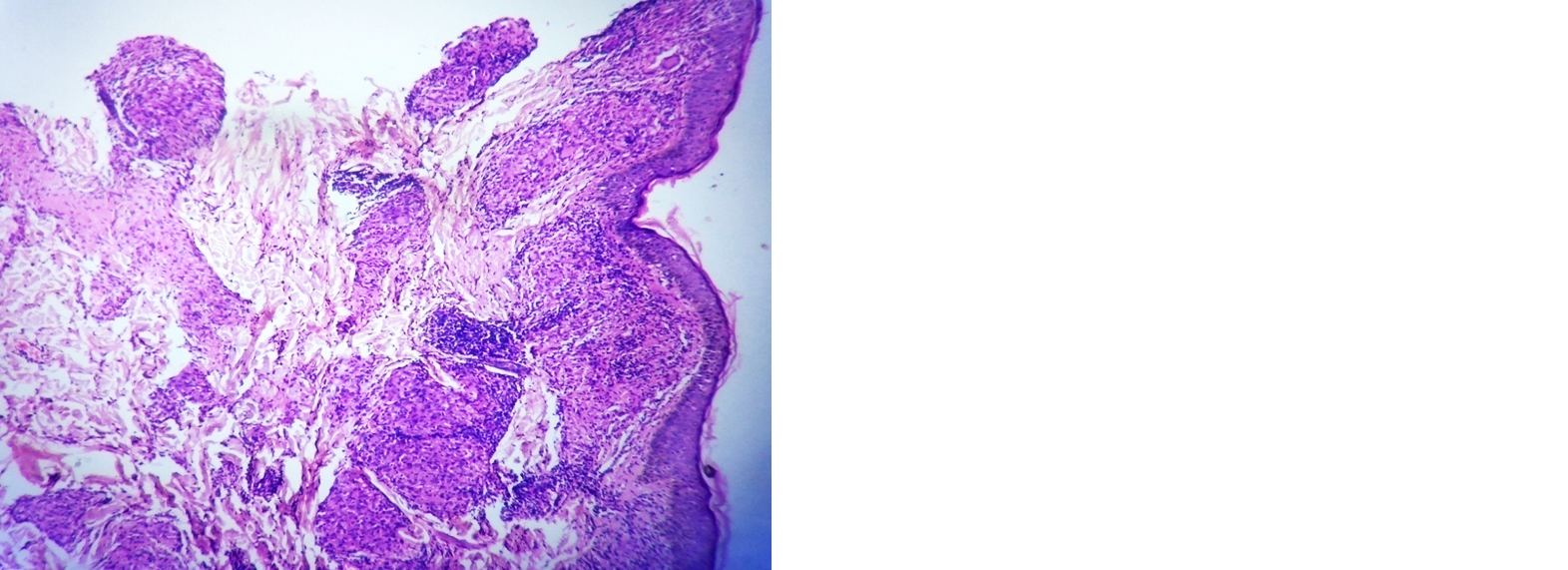
Figure 1: Borderline tuberculoid–dermal granuloma hugging the epidermis, H and E; 4×.
Tuberculoid leprosy: Atrophy in epidermis, dermis showed granulomas with giant cells in majority of cases, lymphocyte and macrophage infiltration around neurovascular bundle, adnexal structures and arrector pilae also noted. Fite Faraco stain was negative in all the cases (Figure 2, Table 4).
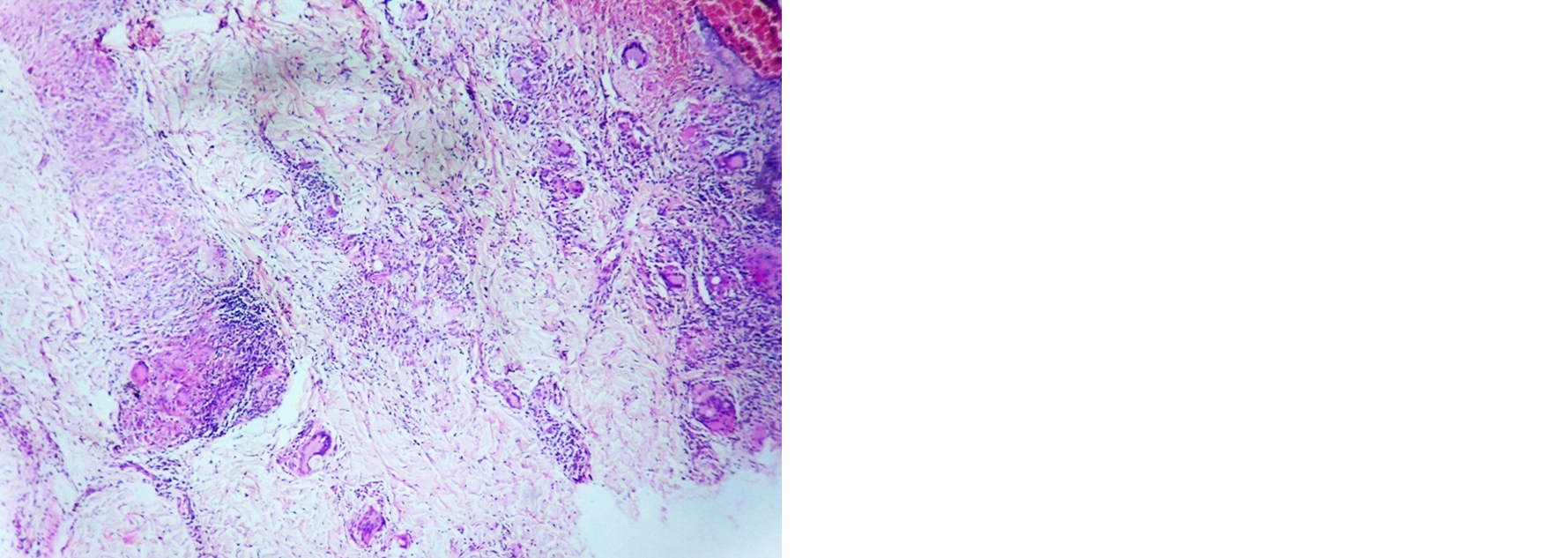
Figure 2: Tuberculoid leprosy– dermis showing granuloma with giant cells, H and E; 4×.
Indeterminate leprosy: Atrophy in epidermis, dermis showed grenz zone, lymphocyte infiltration around neurovascular bundle, adnexal structures and arrector pilae in majority of the cases and macrophage infiltration around neurovascular bundle, adnexal structures and arrector pilae in few of the cases were also noted. Fite Faraco stain was negative in all the cases (Figure 3, Table 4).
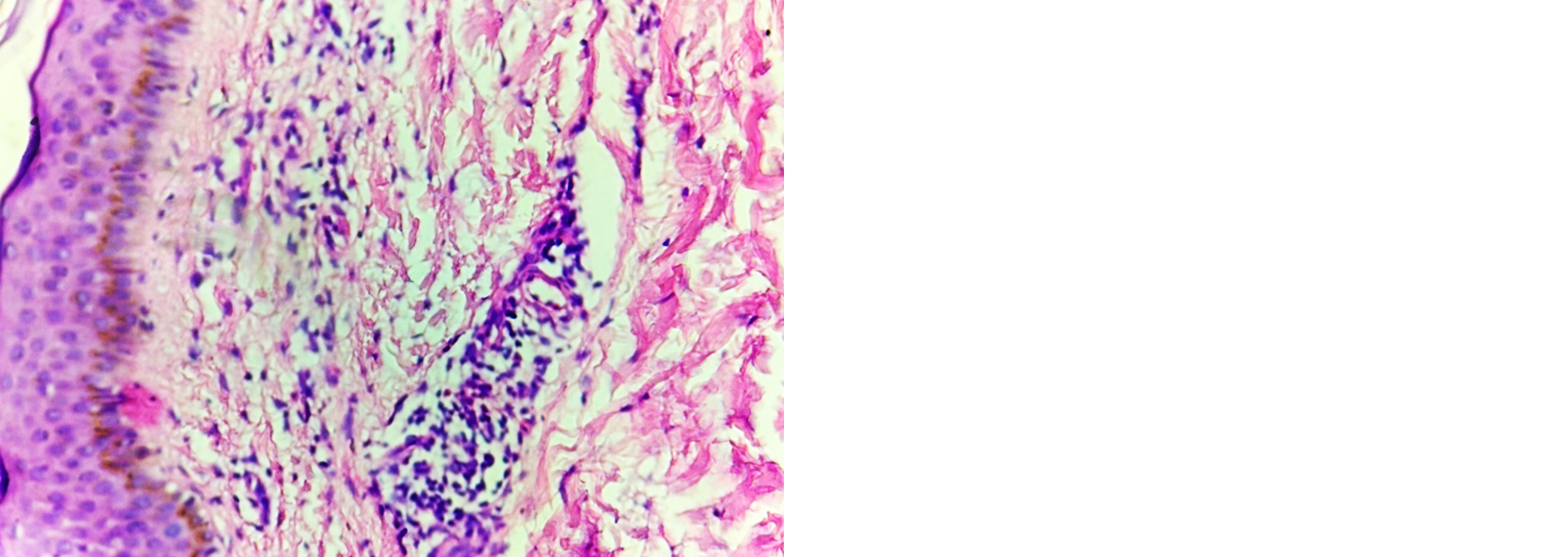
Figure 3: Indeterminate leprosy–dermis showing lymphocyte infiltration around blood vessels, H and E; 4×.
Table 5: Clinicohistopathological correlation.
|
Clinical diagnosis
|
No of cases
|
Histopathology
|
|
Indeterminate
|
Borderline tuberculoid
|
Tuberculoid
|
No evidence of leprosy
|
|
Tuberculoid
|
7
|
2
|
1
|
1
|
3
|
|
Borderline tuberculoid
|
63
|
26
|
20
|
12
|
5
|
|
Indeterminate
|
4
|
4
|
-
|
-
|
-
|
|
Not clinically classified
|
34
|
11
|
12
|
4
|
7
|
|
Relapses
|
20
|
7
|
6
|
2
|
5
|
|
Borderline
|
1
|
-
|
1
|
-
|
-
|
|
Type 2
|
1
|
1
|
-
|
-
|
-
|
Table 5 shows the evaluation of the concordance between the clinical classification (diagnostic suspicion) and histopathological classification of the 130 patients. According to clinical diagnosis 63 cases were diagnosed as borderline tuberculoid; on histopathology 20 cases were borderline tuberculoid and remaining 26 indeterminate, 12 tuberculoid and 5 cases were diagnosed with no evidence of leprosy. Out of 7 cases diagnosed as tuberculoid leprosy clinically, one case turned to be tuberculoid on histopathology remaining 2, 1 and 3 were indeterminate, borderline tuberculoid and no evidence of leprosy respectively. One case of mid borderline leprosy turned out to be borderline tuberculoid on histopathology. One case diagnosed as Type 2 reaction clinically was indeterminate leprosy on histopathology. The cases diagnosed as indeterminate leprosy clinically correlated with histopathology. 34cases were not classified clinically but on histopathology 11, 12, 4 and 7 cases were indeterminate, borderline tuberculoid, tuberculoid and no evidence of leprosy respectively. 20 cases were relapse cases clinically, but on histopathology 7, 6, 2 and 5 cases were diagnosed as indeterminate, borderline tuberculoid, tuberculoid and no evidence of leprosy respectively.
Discussion
Leprosy, also known as Hansen’s disease, is a chronic granulomatous infectious disease that primarily affects the skin and the peripheral nerves. The clinical and pathological features reflect the cell mediated immunity of the host which needs an appropriate classification because of its varied manifestations [6]. Leprosy is classified within two poles of the disease with transition between the clinical forms [7]. The WHO classification of dividing leprosy into paucibacillary (<5 lesions) and multibacillary (≥5 lesions) is recommended for routine use and either Indian or Ridley-Jopling classification for research workers [8]. The correct classification of leprosy cases is an important tool for the proper allocation of patients in the multidrug therapy program, since the duration of treatment and dosage of medication used differ between the paucibacillary and multibacillary forms [9]. The widely accepted Ridley and Jopling classification is based on clinical, histopathological and immunological features [10]. The main drawback of Ridley-Jopling classification is that there is no specific place for the indeterminate and pure neuritic leprosy in the spectrum. There can be clinicohistopathological discordance that may cause confusion under this classification [11].
The diagnosis of leprosy is based on different clinical parameters which involve detailed examination of skin lesions and peripheral nerves along with slit skin smear examination, histopathological examination, and demonstration of acid fast bacilli [12]. The terms multibacillary and paucibacillary classification is to be based on the number of skin lesions, less than or equal to five for paucibacillary and greater than five for the multibacillary form [1, 8].
The study included 130 patients ranging from 4 to 80 years which showed slightly higher preponderance in males (M:F– 1.6:1). Though majority patients were young adult and middle aged (50 and 39), 17.6% were children up to 15 years of age. Clinical spectrum of leprosy cases in the present study revealed maximum cases in borderline tuberculoid followed by tuberculoid and indeterminate cases. Others with diagnoses included not classifiable, relapses, borderline and reactions were also considered depending on the number of lesions.
In the present study, majority of the patients presented with hypopigmented patch and nerve thickening followed by erythematous patch and trophic ulcers and fewer with deformities. On histopathology we found indeterminate form was the predominant type of paucibacillary leprosy. The studies by Narasimha Rao et al [13] and Veena et al [14] in their study observed borderline tuberculoid leprosy to be predominant type in histopathological diagnosis.
Some degree of overlap between different types of leprosy clinically as well as histopathologically will be present and there is always chance of inter observer variation for both clinician and pathologist as well [15]. In our study clinically majority of the cases were diagnosed borderline tuberculoid leprosy, but in histopathology majority of the cases turned out to be indeterminate leprosy.
Indeterminate leprosy is an early and transitory stage of leprosy characterized by one or more hypopigmented macules. Indeterminate leprosy may evolve over a period of 2–5 years and may spontaneously resolve or establish into one of the subtypes in the spectrum of leprosy [16].
Conclusion
Tissue diagnosis play a significant role in the diagnosis of leprosy. Clinical evaluation and skin smear examination is required for early diagnosis and treatment, but in some early and borderline cases of leprosy it is difficult to label only on clinical basis, so histopathological evaluation is must for confirming the diagnosis in doubtful cases of leprosy. Pathological examination helps to confirm a presumptive clinical diagnosis and also aids in extra typing.
Acknowledgement
Department of Pathology and Dermatology, KIMS, Hubli, Karnataka, India.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Bhat RM, Prakash C. Leprosy: An overview of pathophysiology. Interdisciplinary Perspectives on infectious diseases. 2012:pp1–6.
[2] World Health Organization. Guide to elimination of leprosy as a public health problem, 1st Ed. Geneva; 2000.
[3] Shivamurthy V, Gurubasavaraj H, Shashikala PS, Kumar P. Histomorphological study of leprosy. Afr J Med Health Sci. 2013; 12(2):68–73.
[4] National leprosy eradication program - progress report for the Year 2014-15. Central Leprosy Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, New Delhi, Accessed on February 5, 2016. Available from: http//w ww.nlep.nic.in/pdf/report
[5] Sharma A, Sharma RK, Goswsami KC, Bardwaj S. Clinico-histopathological correlation in leprosy. JK Science. 2008; 10(3):120–123.
[6] Thakkar S, Patel SV. Clinical profile of leprosy patients: A prospective study. Indian J Dermatol 2014; 59:158–162.
[7] Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system, Int J Lepr Other Mycobact Dis. 1966; 34(3):255–273.
[8] Sachdeva S, Amin SS, Khan Z, Alam S, Sharma PK. Childhood leprosy: A retrospective study. J Public Health Epidemiol. 2010; 2:267–271.
[9] Santos VS, Neto PTDM, Raposo OFF, Fakhouri R, Reis FP, et al. Evaluation of agreement between clinical and histopathological data for classifying leprosy. Int J Infect Dis. 2013; 17(3):e189–e192.
[10] Suri SK, Iyer RR, Patel DU, Bandil S, Baxi S. Histopathology and clinico-histopathological correlation in Hansen’s disease. J Res Med Den Sci. 2014; 2(1):37–44.
[11] Arif T, Dorjay K, Adil M, Sami M. Classification of leprosy – From past to present. J Pakistan Assoc Dermat. 2018; 28(1):95–99.
[12] Semwal S, Joshi D, Goel G, Asati D, Kapoor N. Clinico-histological correlation in Hansen’s disease: Three-year experience at a newly established tertiary care center in central India. Indian J Dermatol. 2018; 63:465–468.
[13] Rao PN, Pratap DVS, Ramana Reddy AV, Suneetha S. Evaluation of leprosy patients with 1 to 5 skin lesions with relevance to their grouping into paucibacillary or multibacillary disease. Indian J Dermatol Venereol Leprol. 2006; 72(3):207–210.
[14] Veena S, Kumar P, Shashikala P, Gurubasavaraj H, Chandrasekhar HRM. Significance of histopathology in leprosy patients with 1-5 skin lesions with relevance to therapy. J Lab Physicians. 2011; 3(1):21–24.
[15] Bommakanti J, Putta S, Gokhale S. Histopathological relevance in clinical spectrum of Hansen’s disease. JMSCR 2016; 4(12):14678–14684.
[16] Narang T, Kumar B. Leprosy in children. Indian J Paediatr Dermatol. 2019; 20(1):12–24.