Full Text
Introduction
According to GLOBOCAN 2020, breast cancer is the most common cancer worldwide. In India, approximately 1.78 lakh new cases of breast cancer were diagnosed in 2020 (13.5% of total cases). It is the leading cause of death due to cancer in India (10.6%) and fourth worldwide (6.9%) [1].
Patients with locally advanced breast cancer (LABC) require multidisciplinary team approach, that incorporates surgery, chemotherapy, radiation therapy, biologic and hormonal therapies, in varying combination [2-4]. Large prospective trials and a meta-analysis have shown that adjuvant chest wall radiotherapy improves local control and survival in node positive breast cancer patients [5, 6]. Chest wall irradiation is commonly done with tangential beams which include part of the anterior thoracic cavity, thereby potentially affecting the lung and heart and leading to higher risk of cardiac morbidity [6]. This becomes even more complicated as most of the chemotherapeutic agents used to treat breast cancer like anthracyclines, trastuzumab, possess cardiotoxic potential.
Intensity Modulated Radiation Therapy (IMRT) has proved to be superior than 3-Dimensional Conformal Radiation Therapy (3DCRT) in various sites like head and neck, central nervous system, lung, prostate to prescribe maximum dose to the target with minimum dose to critical organs at risk. IMRT directs radiation at the breast tumour and modulates the intensity of the radiation beams with better accuracy, helping to spare healthy tissue surrounding the breast tumour [7]. But on the other hand, IMRT increases integral dose to normal healthy tissues, increasing concern about second malignancy in long term survivors.
Most of the studies from Western world have been performed on whole breast radiotherapy after breast conservative surgery; the data on post-mastectomy radiation therapy is scarce. So, our study was carried out to compare the dosimetry and acute toxicity profile of 3DCRT and IMRT in post-mastectomy patients.
Materials and methods
It was a double arm, single institutional, prospective, comparative study among post-modified radical mastectomy female patients of locally advanced carcinoma breast aged between 20-70 years having adequate hepatic, renal, haematological parameters and an ECOG score of 0-2. Patients with bilateral, recurrent or metastatic breast carcinoma, previous history of any other malignancy or radiotherapy were excluded. This study was done between March 2020 and August 2021. Approval was taken from Institutional Ethical Committee and informed consent was taken from every participant.
Study technique
Patients were randomized into two groups-
Arm A (Study arm): Received radiotherapy with IMRT technique at a dose of 50 Gy in 25 fractions, 2 Gy/fraction, 5 days per week for total 5 weeks.
Arm B (Control arm): Received radiotherapy with 3DCRT technique at a dose of 50 Gy in 25 fractions, 2Gy/fraction, 5 days per week for total 5 weeks.
Radiotherapy technique
Patient positioning: Supine position on breast board (10º - 30º) to get longitudinal axis of sternum parallel to the radiation couch. Arms are in the abducted and externally rotated position above their head with holding the hand grips. Neck is extended on a suitable head-rest.
Immobilisation: After proper positioning of the patient, a 1cm wax bolus is kept over the chest wall followed by a thermoplastic mask customised to individual patient’s chest wall. Simulation- A non-contrast CT simulation was done with proper positioning. Radio-opaque wires used to mark the mastectomy scar and the clinical boundaries.
Contouring and planning: Delineation of target volumes and organ at risk (OAR) was done on the basis of planning CT scan as per contouring guideline. Clinical Target Volume (CTV) included ipsilateral chest wall and supraclavicular and axillary lymph nodes as clinically indicated.
PTV was created adding5mm margin to CTV. Lung, heart, spinal cord, thyroid gland, esophagus and contralateral breast were contoured as organs at risk.
IMRT planning was done by five to seven non-coplanar beams to adequately cover the planning target volume (PTV), while minimizing the dose to ipsilateral lung, heart, contralateral breast.
3DCRT planning was done by two tangential semi-opposed beams (to avoid divergence), physical wedges (usually 15° or 30°) and multi-leaf collimator. The beam angles, wedge angles, and beam weighting (usually minimal) were chosen to optimize coverage of the PTV, while minimizing exposure to OARs.
Treatment delivery: Treatment plans were generated using Eclipse treatment planning system. Treatment was delivered using TRUE BEAM machine (VARIAN, Version 15.6) with 10MV energy beam.
Dosimetric evaluation: Data collection included the volume of PTV receiving greater than 95% to 107% of prescribed dose (V95 and V107); the dose delivered to 98% (Dnear-min, D98) and 2% (Dnear-max, D2) of the volume of PTV; and mean dose of the PTV (Dmean) from the dose-volume histogram (DVH).
Dose homogeneity index (HI) and Conformity Index (CI) were calculated according to definition proposed by the International Commission on Radiation Units and Measurements (ICRU) Report 83.
Homogeneity index (HI) = (D2 - D98) / Dp [Dp is the prescribed dose]
Conformity index (CI) = V(RI) / TV [V(RI) is the reference isodose volume and TV is the target volume].
Evaluation of organ at-risk
Lung: Percentage volume of ipsilateral lung receiving 5 Gy (V5), 20 Gy (V20), and the mean lung dose (Dmean) was calculated.
Heart: Percentage of volume receiving 5 Gy (V5), 25 Gy (V25) and the mean heart dose (Dmean) was calculated.
Maximum dose (Dmax) was calculated for spinal cord and mean dose (Dmean) was calculated for thyroid and oesophagus.
All patients were followed up for treatment related acute toxicity during the entire course of treatment and then at every month for first three months and then 3 monthly for 6 months with at least 6 months of follow-up for each patient after completion of treatment. Treatment related toxicities were assessed with common terminology criteria for adverse events scale (CTCAE v5.0) and Radiation therapy oncology group (RTOG) scoring. The Figure 1 shows consort flow diagram.
Statistical analysis
Data was analysed and compared according to appropriate statistical tests using SPSS V.24 software and Microsoft word-excel and GraphPad prism. Significance of dose distribution was statistically evaluated using non-parametric statistical methods. Any p value < 0.05 was considered statistically significant.
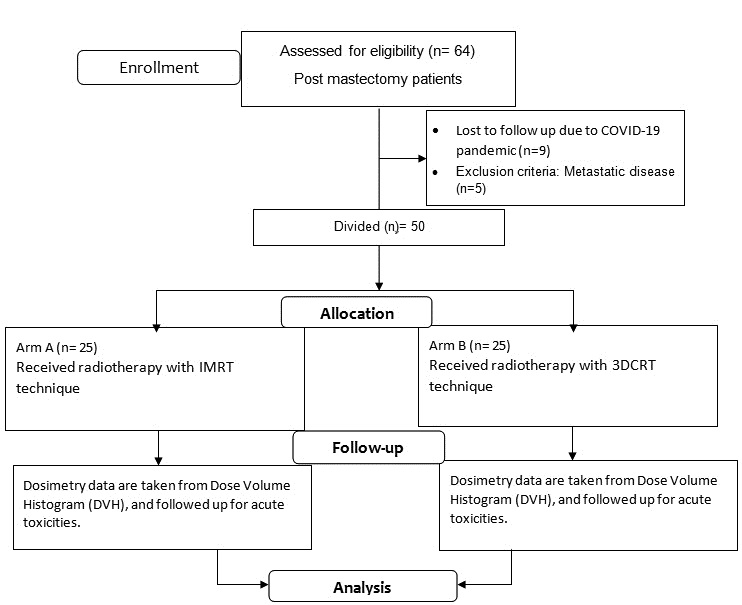
Figure 1: Consort flow diagram.
Results
Baseline characteristics
Baseline characteristics of patients like age, residence, education level, side of the disease, tumour (T) stage, nodal (N) stage at presentation and performance status were comparable between both the arms of the study (Table 1).
Table 1: Distribution of baseline characteristics between two study arms.
|
Characteristics
|
Arm of the study
|
P value
|
|
Study arm (n=25)
|
Control arm (n=25)
|
|
Mean age of patients (In years)
|
48.08
|
45.6
|
0.144
|
|
Residence
|
Urban
|
7
|
11
|
0.24
|
|
Rural
|
18
|
14
|
|
Tumour (T) stage
|
T2
|
1
|
2
|
0.46
|
|
T3
|
13
|
16
|
|
T4
|
11
|
7
|
|
Nodal stage
|
N1
|
14
|
14
|
1
|
|
N2
|
11
|
11
|
|
Laterality of disease
|
Right
|
18
|
13
|
0.15
|
|
Left
|
7
|
12
|
|
Performance status (ECOG score)
|
0
|
8
|
9
|
0.59
|
|
1
|
16
|
16
|
|
2
|
1
|
0
|
Dose distribution evaluation
A. PTV parameters - D2, D98 and Dmean
The mean of D2 value of IMRT and 3DCRT were 52.92 Gy and 54.45 Gy respectively. IMRT has statistically significant lower value than arm B (p value <0.0001).
The mean of D98 value of IMRT and 3DCRT were 45.46±1.85 Gy and 41.50±3.08 Gy respectively. IMRT has statistically significant higher value than arm B (p value <0.0001). The mean value of Dmean for IMRT and 3DCRT were not significantly different (50.17 Gy vs 50.29 Gy, p value 0.32) (Table 2).
Table 2: Planning target volume (PTV) parameters (D2, D98, Dmean).
|
Category
|
Descriptive statistical parameter
|
IMRT (N = 25)
|
3DCRT (N = 25)
|
P value
|
|
D2
|
Mean ± SD
|
52.92 ± 1.13
|
54.45 ± 0.89
|
<0.0001
|
|
Min-Max
|
51.1 – 56.4
|
52.8 – 57.8
|
|
Median
|
52.9
|
54.4
|
|
D98
|
Mean ± SD
|
45.46 ± 1.85
|
41.50 ± 3.08
|
<0.0001
|
|
Min-Max
|
40.75 – 49.54
|
28.9 – 45.89
|
|
MEDIAN
|
45.9
|
42
|
|
Dmean
|
Mean ± SD
|
50.17 ± 0.67
|
50.29 ± 0.71
|
0.32
|
|
Min-Max
|
49.14 – 51.9
|
49.7 – 53
|
|
Median
|
50
|
50
|
B. PTV parameters - V95 AND V107
The mean value of V95 for IMRT was 92.78 and for 3DCRT it was 85.02. IMRT had statistically significant higher value than 3DCRT (p value <0.0001).
The mean of V107 of IMRT and 3DCRT were 2.33 and 10.26 respectively. IMRT had significantly lower value (p value <0.0001) (Table 3).
Table 3: PTV parameters (V95, V107).
|
Category
|
Descriptive statistical parameter
|
IMRT
(N = 25)
|
3DCRT
(N = 25)
|
P value
|
|
V95
|
Mean ± SD
|
92.78 ± 3.36
|
85.02 ± 4.53
|
<0.0001
|
|
Min-Max
|
86.54 – 99.97
|
73.04 – 95
|
|
Median
|
93.1
|
85.9
|
|
V107
|
Mean ± SD
|
2.33 ± 2.89
|
10.26 ± 7.35
|
<0.0001
|
|
Min-Max
|
0 – 9.33
|
0.35 – 38.69
|
|
Median
|
0.79
|
10.38
|
C. PTV parameters - Homogeneity index (HI)
The mean of HI value of IMRT and 3DCRT were 0.14 and 0.26 respectively. The difference was statistically significant (p value <0.0001) (Table 4).
Table 4: PTV parameters –HI.
|
Category
|
Descriptive statistical parameter
|
IMRT
(N = 25)
|
3DCRT
(N = 25)
|
P value
|
|
HI
|
Mean ± SD
|
0.14 ± 0.04
|
0.26 ± 0.06
|
<0.0001
|
|
Min-Max
|
0.06 – 0.24
|
0.20 - 0.51
|
|
Median
|
0.14
|
0.24
|
D. PTV parameters- Conformity index (CI)
The mean value of CI for IMRT was 0.94 and for 3DCRT it was 0.74. IMRT had statistically significant higher CI than 3DCRT (p value 0.0028) (Table 5).
Table 5: Comparison of ci between IMRT and 3DCRT.
|
Category
|
Descriptive statistical parameter
|
IMRT
(N = 25)
|
3DCRT
(N = 25)
|
P value
|
|
CI
|
Mean ± SD
|
0.94 ± 0.19
|
0.74 ± 0.24
|
0.0028
|
|
Min-Max
|
0.65 – 1.53
|
0.32 – 1.17
|
|
Median
|
0.9
|
0.72
|
Evaluation of OAR dosimetry
A. Dose to OAR- Ipsilateral lung doses
The mean of ipsilateral lung V5 of IMRT and 3DCRT were 92.83 and 52.05 respectively. Ipsilateral lung V5 of IMRT was significantly higher than 3DCRT (p value <0.0001).
The mean value of ipsilateral lung V20 and Dmean were not significantly different among the IMRT and 3DCRT arms (p value 0.12 and 0.98 respectively) (Table 6).
Table 6: Ipsilateral lung dose.
|
Category
|
Descriptive statistical parameter
|
IMRT (N = 25)
|
3DCRT (N = 25)
|
P value
|
|
V5
|
Mean ± SD
|
92.83 ± 8.55
|
52.05 ± 12.18
|
<0.0001
|
|
Min-Max
|
73.5 – 100
|
26.63 – 80
|
|
Median
|
97.32
|
54.39
|
|
V20
|
Mean ± SD
|
31.44 ± 4.32
|
35.34 ± 8.87
|
0.12
|
|
Min-Max
|
19.55 – 37.9
|
18 – 49.2
|
|
Median
|
31.78
|
35.23
|
|
DMEAN
|
Mean ± SD
|
19.13 ± 2.74
|
18.79 ± 4.79
|
0.98
|
|
Min-Max
|
10.89 – 23.29
|
9 – 31.06
|
|
Median
|
19.22
|
19.8
|
B. Dose to OAR - Heart doses in left sided breast cancer
The mean value of heart V5 of IMRT and 3DCRT were 98.47 and 43.82 respectively. This difference was statistically significant (p value <0.0001).
The mean value of heart V25 in left sided disease was significantly lower in IMRT arm than 3DCRT arm (22.59 and 25.64, p value 0.01).
The mean value of heart Dmean in left sided disease was comparable between IMRT and 3DCRT (14.72 Gy vs 16.38 Gy, p value 0.62) (Table 7).
Table 7: Heart dose in left sided breast carcinoma.
|
Category
|
Descriptive statistical parameter
|
IMRT
(N = 7)
|
3DCRT
(N = 12)
|
P value
|
|
V5
|
Mean ± SD
|
98.47 ± 2.75
|
43.82 ± 13.04
|
<0.0001
|
|
Min-Max
|
92.62 – 100
|
27.89 – 76.1
|
|
Median
|
100
|
41.32
|
|
V25
|
Mean ± SD
|
22.59 ± 2.36
|
25.64 ± 2.77
|
0.01
|
|
Min-Max
|
17.81 – 25
|
19.22 – 29.43
|
|
Median
|
22.5
|
25.5
|
|
DMEAN
|
Mean ± SD
|
14.72 ± 1.29
|
16.38 ± 3.98
|
0.62
|
|
Min-Max
|
13.49 – 17.37
|
10.55 – 23.6
|
|
Median
|
14.27
|
15.8
|
C. Dose to OAR- Heart doses in right sided breast cancer
The mean value of heart V5 in right sided disease had a much higher value in IMRT than 3DCRT (76.93 vs 6.91). The difference was statistically significant (p value <0.0001).
The mean value of V25 for right sided disease was comparable between IMRT and 3DCRT are (4.04 vs 2.16, p value 0.1).
The mean value of heart Dmean in right sided disease was significantly higher for IMRT than 3DCRT (8.54 Gy vs 2.18 Gy, p value <0.0001).
D. Dose to OAR- Spinal cord, thyroid, esophagus, contralateral breast
The mean value of spinal cord Dmax of IMRT was significantly lower than 3DCRT (26.79 Gy vs 40.52 Gy, p value <0.0001).
The mean Dmax of thyroid and Dmean of esophagus was not significantly different between IMRT and 3DCRT (p value 0.42 and p value 0.17). Mean Dmax of oesophagus in IMRT and 3DCRT was also comparable (40.15 Gy vs 47.79 Gy,p value 0.06).
Mean Dmean of contralateral breast in IMRT arm was significantly higher than 3DCRT arm (2.61 vs 1.16, p value 0.02).
E. Acute toxicities - Acute dermatitis, acute pneumonitis, acute hematological toxicity
Though numerically grade 3 acute skin toxicity was more in IMRT arm, the difference was not statistically significant (p value 0.43).
There is no statistically significant difference related to acute lung and haematological toxicities in both the arms (p value 0.25 and p value 0.74).
Discussion
Several studies demonstrated dosimetric benefit of IMRT compared to 3DCRT for whole breast radiotherapy in early breast cancer patients but for post mastectomy chest wall irradiation, such data is scarce. Fiorentino et al. compared 3DCRT and 4-fields IMRT treatment plans, in term of target dose coverage, integral dose and dose to OARs in early breast cancer and concluded 4-fields IMRT technique significantly reduced the dose to OARs and normal tissue, with a better target coverage compared to 3DCRT [8]. We conducted this study to compare these two techniques in post-mastectomy radiation therapy (PMRT).
Comparing the dose distribution parameters of the PTV, near-maximum dose (D2) and near-minimum dose (D98) were better in IMRT than 3DCRT and they were statistically significant with p value of <0.0001. The volume of PTV receiving 95% (V95) and 107% (V107) were also significantly better in IMRT than 3DCRT (p value of <0.0001). But, mean dose (Dmean) was comparable in both the techniques (p value 0.32).
We observed significantly better homogeneity index in IMRT with mean value of 0.14 than 3DCRT with mean value of 0.26 (p value <0.0001). Similar result has reported by Beckham et al. with p value of <0.05 [9]. But no significant difference is noted by Moorthy et al., Rudat et al. and Li et al. [10-12].
Conformity index is also significantly better in IMRT with mean value of 0.94 compared to 0.74 in 3DCRT (p value 0.003) reflecting more conformal dose distribution in IMRT. Similar result is reported by Beckham et al., Moorthy et al., and Rudat et al. [9-11].
Radiation pneumonitis is one of the most common side effects following post mastectomy radiotherapy. For the patients treated with 3DCRT, the volume of lung receiving 20 Gy (V20) has been found to predict the risk of symptomatic radiation pneumonitis [13-15]. In our study, the V20 was numerically lower in IMRT (mean 31.44) than 3DCRT (mean 35.34) but not statistically significant (p value 0.12). Study by Beckham et al. and Moorthy et al. showed significantly lower V20 for lung in IMRT than 3DCRT [9, 10]. Mean dose to lung (Dmean) in our study was similar for IMRT and 3DCRT (p value 0.98). The volume of lung receiving 5Gy (V5) is significantly higher in IMRT than 3DCRT (p value <0.0001) in our study. Similar result has obtained in the studies by Beckham et al. and Li et al. [9, 12]. No patient in both the arms shows acute radiation pneumonitis ≥ RTOG grade 2 but grade 1 radiation pneumonitis was numerically higher in IMRT than 3DCRT (p value 0.25).
Higher cardiac morbidity and mortality following PMRT has always been an area of significant concern. It has been established that adjuvant radiotherapy to left sided breast cancer has a small but significant increase in the risk of both cerebrovascular and cardiovascular deaths [16-18]. However, development of cardiotoxicity is a complex phenomenon and depends on a number of conditions like pre-existing cardiovascular disease, obesity, hypertension, smoking, old age and use of cardiotoxic chemotherapeutic agents. Therefore, it is desirable to minimize the irradiated heart volume to the greatest possible extent without compromising the target volume. In our study, the volume of heart receiving 25 Gy dose (V25) was significantly lower in IMRT than 3DCRT (mean value 22.59 vs 25.64) for the left sided breast cancer patients (p value 0.01). Similarly, Moorthy et al. showed statistically significant lower V25 heart dose in IMRT [10]. Although statistically not significant, mean dose to heart (Dmean) was numerically lower in IMRT than 3DCRT (mean value 14.72 Gy vs 16.38Gy, p value 0.62). But the low dose volume (V5) of heart was significantly higher in IMRT than 3DCRT (p value <0.0001). This result is comparable with the result reported by Beckham et al. [9]. For right sided breast cancer, V5 and Dmean in IMRT were significantly higher than 3DCRT with p value of <0.0001.
In both heart and lung dosimetry, IMRT had better sparing effect but it irradiated a larger volume of organ to a very low dose than 3DCRT.This low volume bath is a drawback for IMRT when we consider the stochastic effects of radiation, as it may increase the chances of second malignancies and late dysfunctions. This basic fact was also echoed in this study results.
In this study, maximum dose to spinal cord (Dmax) is significantly lower in IMRT than 3DCRT (mean value 26.79 Gy vs 40.52 Gy with p value of <0.0001). Maximum dose to thyroid gland and oesophagus (Dmax) were comparable in both the arms with p value of 0.42 and 0.06). Mean dose to contralateral breast (Dmean) is significantly higher in IMRT (mean value 2.61Gy) than 3DCRT (mean value 1.16Gy) with p value of 0.02.
So, for post-mastectomy radiotherapy to chest wall, IMRT significantly improves the conformity and homogeneity of the plan and reduce the high dose volume of the OARs compared to 3DCRT.
Limitation
Our sample size was small, so any statistical data has to be interpreted with caution. It is a single institutional study; hence result derived cannot be extrapolated on entire population. Entire study duration was about 18 months including patient accrual, intervention and assessment. So, the late toxicity and the loco-regional control and survival could not be assessed. In analysis, subjective variation of treatment planning by doctors and physicist cannot be adjusted accordingly. Long term follow up will be done further to compare late radiation related toxicities, 5 year OS (overall survival) and PFS (Progression free survival) between 3DCRT and IMRT.
Conclusion
To conclude, it can be said that in case of post-mastectomy chest wall irradiation IMRT has better planning target volume coverage than 3DCRT with more homogenous and conformal plans. To spare the organs at risk, IMRT is more efficient than 3DCRT in high dose volume. But, further studies with large sample size and longer duration of follow up is necessary for defining an ideal radiotherapy technique with special emphasis on long term disease control and treatment related late toxicities.
Conflicts of interest
Author declares no conflicts of interest.
References
[1] Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020; 68:394–424.
[2] Goyal S, Buchholz TA, Haffty BG. Breast cancer: early stage. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Principles and practice of radiation oncology. Philadelphia: Lippincott Williams & Wilkins 2013; 6th edn. pp.1044–1140.
[3] Buchholz TA, Wazer DE, Haffty BG. Breast cancer: locally advanced and recurrent disease, postmastectomy radiation, systemic therapies. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Principles and practice of radiation oncology. Philadelphia: 6th edn Lippincott Williams & Wilkins 2013; pp.1140–1164.
[4] Sainsbury R. The Breast. In: Williams NS, Bulstrode CJK, O’connell PR, editors. Bailey & Love’s Short Practice of Surgery. Boca Raton: CRC Press Taylor & Francis Group 2013; 26th edn. pp.798–819.
[5] Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007; 82(3):247–253.
[6] Clarke M, Collins R, Darby S, Davies C, Elphinstone P, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366(9503):2087–2106.
[7] Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, et al. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010, 28(21):3437–3441.
[8] Fiorentino A, Ruggieri R, Giaj-Levra N, Sicignano G, Paola GD, et al. Three-dimensional conformal vs intensity modulated radiotherapy in breast cancer treatment: is necessary a medical reversal? Radiol Med. 2017; 122(2):146–153.
[9] Beckham WA, Popescu CC, PatenaudeW, Wai ES, Olivotto IA. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys. 2007; 69(3):918–924.
[10] Moorthy S, Sakr H, Hasan S, Samuel J, Al-Janahi S, et al. Dosimetric study of SIB-IMRT versus SIB-3DCRT for breast cancer with breath-hold gated technique. Int J Cancer Ther Oncol. 2013; 1:101–110.
[11] Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K, Altuwajri S. Tangential beam IMRT versus tangential beam 3DCRT of the chest wall in post-mastectomy breast cancer patients: a dosimetric comparison. Radiat Oncol 2011; 6:26.
[12] Li W, Wang J, Cheng H, Yu H, Ma J. IMRT vs 3DCRT for post-mastectomy irradiation of chest wall and regional nodes: a population-based comparison of normal lung dose and radiation pneumonitis. Int J Clin Exp Med. 2016; 9(11):22331–22337.
[13] Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002; 52(5):1196–1206.
[14] Kahan Z, Csenki M, Varga Z, Szil E, Cserháti A, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2007; 68(3):673–681.
[15] Marks LB, Bentzen SM, Deasy JO, Kong FMS, Bradley JD, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010; 76(3Suppl):S70–76.
[16] Taylor CW, McGale P, Darby SC. Cardiac risks of breast-cancer radiotherapy: a contemporary view. Clin Oncol. 2006; 18(3):236–246.
[17] Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010; 76(3):656–665.
[18] Hooning MJ, Botma A, Aleman BM, Baaijens MHA, Bartelink H, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. Int J Radiat Oncol Biol Phys. 2007; 99(5):365–375.