Review
2014
June
Volume : 2
Issue : 2
Tumor lysis syndrome – An overview and current perspective
Piyush K Jain, Gangadhar Vajrala
Pdf Page Numbers :- 109-112
1Department of Radiation Oncology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad – 500003, AP., India
*Corresponding author: Dr. Piyush K Jain, MBBS, MD., Radiation Oncologist, Department of Radiation Oncology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad – 500003, AP., India. Email: drpkjain82@gmail.com
Received 1 February 2014; Revised 12 March 2014; Accepted 20 March 2014
Citation: Piyush K Jain, Gangadhar Vajrala. Tumor lysis syndrome – An overview and current perspective. J Med Sci Res 2014; 2(2):109-112. DOI: http://dx.doi.org/10.17727/JMSR.2014/2-019
Copyright: © 2014 Piyush K Jain et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Tumor lysis syndrome (TLS) is an oncological emergency where in spontaneous or treatment-induced cell death leads to a constellation of metabolic abnormalities that can result in potentially life threatening complications. It is characterized by hyperkalemia, hyperuricemia, hyperphosphatemia and hypocalcemia. Etiopathogenesis is related to spontaneous cell death of rapidly growing tumor or more commonly to administration of cytotoxic chemotherapy in patients with hematologic malignancies and less so with solid malignancies. The precise incidence of TLS is not known, and neither racial nor gender predilection exists. Approximately 18% of the patients who develop TLS die of its complications [1]. The cornerstone of the management of TLS includes knowledge of its causes, recognition of high-risk patients, vigilant monitoring and prompt initiation of appropriate preventive and therapeutic measures when indicated.
Full Text
Introduction
Tumor lysis syndrome (TLS) is an oncological emergency where in spontaneous or treatment-induced cell death leads to a constellation of metabolic abnormalities that can result in potentially life threatening complications. It is characterized by hyperkalemia, hyperuricemia, hyperphosphatemia and hypocalcemia. Etiopathogenesis is related to spontaneous cell death of rapidly growing tumor or more commonly to administration of cytotoxic chemotherapy in patients with hematologic malignancies and less so with solid malignancies. The precise incidence of TLS is not known, and neither racial nor gender predilection exists. Approximately 18% of the patients who develop TLS die of its complications [1]. The cornerstone of the management of TLS includes knowledge of its causes, recognition of high-risk patients, vigilant monitoring and prompt initiation of appropriate preventive and therapeutic measures when indicated.
Risk factors [2, 3]
1) The presence of high leukocyte count, bulky adenopathy, hepatosplenomegaly or both (i.e. B-cell lymphomas/ leukemias, T-cell lymphomas/ leukemias) often evidenced by elevated pretreatment lactate dehydrogenase (LDH); 2) Tumor highly sensitive to chemotherapy or radiation therapy; 3) Elevated pretreatment uric acid levels; 4) Compromised renal function; 5) Use of potentially nephrotoxic drugs.
Pathophysiology
TLS occurs a few hours or a few days after the initiation of therapy. Cell death leads to release of potassium, phosphate, uric acid, and other purine metabolites (Figure 1) [4-7] jeopardizing the kidney's capacity for clearance with resultant hyperkalemia, hyperphosphatemia, hyperuricemia, and secondary hypocalcemia [8]. Significant increase in serum LDH occurs frequently.
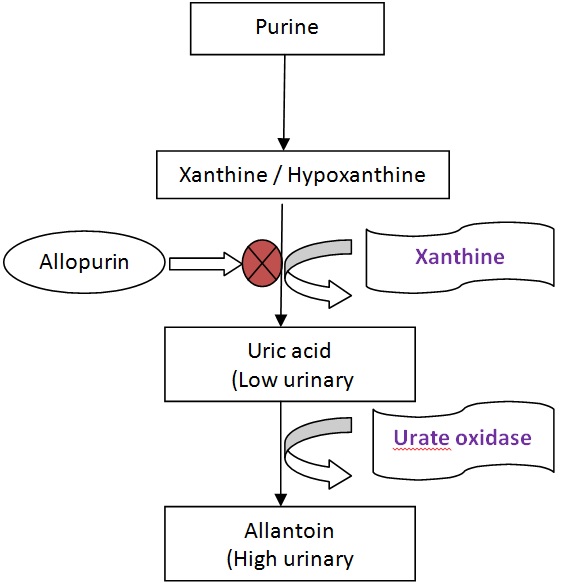
Figure 1: Purine catabolism pathway.
Hyperkalemia poses the greatest immediate threat to the patient with TLS. Release of intracellular potassium from dying cells is the main cause of hyperkalemia. Lowering of adenosine triphosphate (ATP) levels before cell lysis can also lead to leakage of potassium, accounting for the fact that a rise in serum potassium is often the first sign of TLS.
Hyperuricemia is the most common finding of TLS. The real culprit is not the hyperuricemia but the increased renal uric acid excretion, occurring as a result of hyperuricemia, causes the symptoms. When present in high concentrations uric acid crystallizes in the renal parenchyma, distal tubules and collecting ducts where luminal pH is 5.0, leads to intraluminal tubular obstruction and oliguria.
Hyperphosphatemia follows cell lysis, resulting in hyperphosphaturia and hypocalcemia. Hyperphosphaturia enhances the risk of nephrocalcinosis and tubular obstruction. Severe hypocalcemia can complicate hyperkalemia and its associated electrocardiography (ECG) changes as well as hypotension.
Acute renal failure occurring in the setting of TLS is usually multifactorial. Contributing factors include intravascular volume depletion, precipitation of nucleic acid metabolites most notably uric acid and calcium phosphate crystals in the renal tubules (acute nephrocalcinosis).
Diagnosis
The standard definition of TLS comprises of clinical and laboratory definitions. This was originally proposed by Hande & Garrow in 1993, standardized by Cairo & Bishop in 2004, which was later modified by Howard et al in 2011 [9-11] (Table 1).
Table 1: Definition of tumor lysis syndrome (TLS) by Howard et al. 2011.
|
Laboratory TLS
|
Clinical TLS
|
Other
|
|
2 or more of the following metabolic abnormalities occurring simultaneously within 3 days prior to and up to 7 days post -treatment initiation
|
Laboratory –defined TLS accompanied by any one of the following:
|
Any symptomatic hypocalcaemia is diagnostic
|
|
1. Uric acid > 8.0mg/dL (475.8µmol/L) or above upper normal limit for age in children
2. Potassium > 6.0mmol/L
3. Phosphorus >4.5 mg/dL (1.5 mmol/L) or >6.5mg/dL (2.1 mmol/L) in children
4. Corrected calcium #< 7.0mg/dL (1.75 mmol/L) or ionized calcium <1.12 mg/dL (0.3 mmol/L)
|
1. Elevated creatinine level
2. Seizures
3. Cardiac arrhythmias
4. Death
|
|
# corrected calcium in mg/dL = measured calcium level in mg/dL + 0.8 x (4-albumin in g/dL)
|
Risk stratification of patients
Prior to initiation of chemotherapy and/ or steroids patients must be classified into low, intermediate or high risk groups depending on the disease type, WBC count and any additional risk factors as shown in the table 2 [11, 12].
Table 2: Risk stratification of patients with TLS (pre-chemotherapy).
|
Risk group
|
Disease type
|
Additional risk factors*
|
|
High Risk
(>5% risk of tumor lysis)
|
· Burkitts Lymphoma/ Leukemia
· AML or ALL with WCC >50x109/L
|
· Raised LDH (>2x ULN)
· Renal impairment
· Oliguria
· Baseline uric acid >450μmol/l
· Bulky disease >10cm
· Pre-existing TLS
|
|
Intermediate Risk
(1-5% risk of tumor lysis)
|
· AML
· Other ALL
· High grade NHL with bulky disease
· CML accelerated phase/blast crisis
|
|
Low Risk
(<1% risk of tumor lysis)
|
· Myeloma/ CLL
· Hodgkin’s Lymphoma & other NHLs
· CML chronic phase and MPDs
|
|
*Presence of additional risk factors may place patient into a higher risk group.
|
Management
Management of TLS includes preemptive measures and therapeutic measures (Table 3) [10-13].
Preemptive measures
Preemptive measures for the TLS are administered based on the risk-stratification of the patients as shown in tables 2 and 3. These measures should be initiated at least 24 hours prior to chemotherapy.
1) Institution of adequate hydration; 2) Administration of allpurinol; 3) Consideration of Rasburicase in intermediate/ high risk patients; 4) Monitoring of urine output, renal function tests and other labs periodically depending on the risk stratification.
Table 3: Management of TLS based on risk stratification of the patients.
|
Pre-emptive measures
|
Therapeutic measures
|
|
Low risk
|
Intermediate risk
|
High risk
|
Established TLS
|
|
Oral or IV Fluids
|
IV Fluids
|
IV Fluids
|
IV Fluids
|
|
Allopurinol
|
Allopurinol or Rasburicase
|
Rasburicase
|
Rasburicase
|
|
Laboratory tests daily
|
Laboratory tests Q8-12hrs
|
Laboratory tests Q6-8hrs
|
Laboratory tests Q4-6hrs
|
|
|
Inpatient monitoring
|
Cardiac monitoring
|
Cardiac monitoring
|
|
|
|
|
Intensive care unit
|
Therapeutic Measures (for established TLS) [6, 11-13]
1) Aggresive hydration is the single most important intervention. Approximately 3000ml/m2/day is recommended; 2) Alkalinization of urine remains controversial. Although alkalinization is recommended to avoid crystallinization of uric acid, it favors precipitation of calcium/ phosphate complexes in renal tubules, a concern in patients with concomitant hyperphosphatemia. Administration of bicarbonate to achieve alkalinization can worsen the neurologic manifestations of hypocalcemia. Thus alkalinization of urine is not uniformly recommended and in the majority of cases should be avoided; 3) Hyperkalemia should be treated aggressively: Calcium gluconate (10-30 ml of 10% solution intravenously over 5 minutes) antagonizes the cardiac effects of hyperkalemia, Hypertonic dextrose and insulin can shift potassium back into the cells, Loop diuretics can be used to eliminate excess potassium in patients without renal failure, Hemodialysis is indicated in severe cases with renal impairment (K >7.0mmol/L); 4) Hyperphosphatemia and its resultant hypocalcemia: restrict oral intake / iv phosphorus, oral phosphate binders, a) Sevelamer 800 mg-1600 mg PO three times a day with meals - Drug of choice in patients with hypercalcemia, b) Lanthanum carbonate 500-1000 mg PO three times a day with meals, c) Calcium acetate 1334 mg-2668 mg PO three times a day with meals - Avoid in hypercalcemic patients, d) Calcium carbonate1000 mg-2000 mg (elemental calcium) PO three times a day with meals - Avoid in hypercalcemic patients, e) Aluminum hydroxide 300 mg -600 mg PO three times a day with meals - Avoid in patients with renal dysfunction; 5) Hyperuricemia: Allopurinol lowers serum uric acid by inhibiting enzyme xanthine oxidase (Figure 1) but has no effect on preexisting uric acid. Allopurinol should be initiated 24-48 hours prior to chemotherapy. Recommended dose of Allopurinol is 400mg/m2/day orally or 200 - 400 mg/m2/day intravenously. Rasburicase (Recombinant urate oxidase) catalyzes enzymatic oxidation of uric acid into allantoin, which has high urinary excretion. US FDA approved this drug in 2009 based on clinical trial EFC 4978 [14]. Recommended dose is 0.15 - 0.2 mg/kg over 30 minutes once daily for 5 days. It must be administered 4 hours prior to chemotherapy. Uric acid levels are reduced by 86% within 4 hours of rasburicase treatment compared to only 12% in allopurinol treated patients leading to more rapid control [4]. Rasburicase is contraindicated in glucose-6 phosphate dehydrogenase deficient patients, known hypersensitivity reactions, hemolytic anemia or methemoglobinemia. Allopurinol blocks the conversion of xanthines to uric acid reducing the effectiveness of Rasburicase and therefore should not be given together. 6) Uremia (Renal failure): Fluid, electrolyte & blood pressure management, dose adjustment of nephrotoxic drugs, hemodialysis if conservative management fails.
Conclusion
Tumor lysis syndrome is an oncological emergency which can be prevented by early identification of risk factors, clinical, laboratory parameters and prompt initiation of prophylactic measures. Prevention of TLS is the most important step in its management, since it avoids life-threatening complications, unwarranted reduced dosing and/ or delays in chemotherapy. In addition it reduces length of hospital stay and need for dialysis in these patients, thus reducing morbidity, mortality and total treatment.
Acknowledgement
We thank Dr. Bhaskar Rao MD & CEO, KIMS Hospital for his encouragement and support. We would also like to express our gratitude to our fellow & senior radiation oncologists and medical physicists for their constant inspiration and backing.
Conflict of Interest
The authors wish to express that they have no conflict of interest.
References
1. Annemans L, Moeremans K, Lamotte M, Garcia Conde J, van den Berg H, et al. Pan-European multicentre economic evaluation of recombinant urate oxidase (rasburicase) in prevention and treatment of hyperuricaemia and tumour lysis syndrome in haematological cancer patients. Support Care Cancer. 2003; 11(4):249-257.
2. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008; 26(16):2767-2778.
3. Zojer N, Ludwig H. Hematological emergencies. Ann Oncol. 2007; 8 Suppl 1:i45-i48.
4. Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 2001; 97:2998-3003.
5. Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med. 1968; 45:756-779.
6. Klinenberg JR, Goldfinger SE, Seegmiller JE. The effectiveness of the xanthine oxidase inhibitor allopurinol in the treatment of gout. Ann Intern Med. 1965; 62:639-647.
7. Rieselbach RE, Bentzel CJ, Cotlove E, Frei E, Freireich EJ. Uric acid excretion and renal function in the acute hyperuricemia of leukemia. Pathogenic therapeutic uric acid nephropathy. Am J Med 964; 37:872-883.
8. Howard SC, Pui CH. Pitfalls in predicting tumor lysis syndrome. Leuk Lymphoma. 2006; 47(5):782-785.
9. Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematology. 2004; 127:3-11
10. Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011, 364:1844–1854.
11. McBride A, Westervelt P. Recognizing and managing the expanded risk of tumor lysis syndrome in hematologic and solid malignancies. J Hematol Oncol. 2012; 5:75.
12. Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010; 149(4):578-586
13. Larson AL, Pui CH. Tumor lysis syndrome; www.uptodate.com 2011.
14. Ueng S. Rasburicase (Elitek): a novel agent for tumor lysis syndrome. Proc (Bayl Univ Med Cent). 2005; 18(3):275-279.