Original Research
2015
December
Volume : 3
Issue : 4
A study of Acinetobacter from various clinical specimens & its antibiotic sensitivity pattern in a tertiary care hospital
Pragya Rani, Madhavi Latha B, Sukrutha Gopal Reddy, Anil Kumar Bilolikar
Pdf Page Numbers :- 162-165
Pragya Rani1,*, Madhavi Latha B1, Sukrutha Gopal Reddy1 and Anil Kumar Bilolikar1
1Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. Pragya Rani, Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Email: drpragyarani@gmail.com
Received 25 June 2015; Revised 10 August 2015; Accepted 19 August 2015; Accepted 26 August 2015
Citation: Pragya Rani, Madhavi Latha B, Sukrutha Gopal R, Anil Kumar B. A study of Acinetobacter from various clinical specimens & its antibiotic sensitivity pattern in a tertiary care hospital. J Med Sci Res. 2015; 3(4):162-165. DOI: http://dx.doi.org/10.17727/JMSR.2015/3-031
Copyright: © 2015 Pragya Rani, et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Acinetobacter baumannii has emerged as a significant hospital pathogen, quickly becoming resistant to commonly prescribed antimicrobials.
Objectives: To isolate various species of Acinetobacter, to compare inpatients (ICU’s & wards) and outpatients isolates and to know it’s frequency from various clinical specimens.
Material and methods: The retrospective study is conducted in the department of Microbiology, Krishna Institute of Medical Sciences, Secunderabad, from January 2013 to December 2014. The various clinical specimens from inpatients and outpatients were included. The samples were processed as per the standard guidelines. Identification & antibiotic sensitivity testing was done by using GN and AST 281 cards (Vitek 2 compact, BioMerieux) respectively. MIC values of antibiotics were obtained and reporting was done as per the CLSI guidelines. The data was captured from the laboratory computer and analysed.
Results: A total of 496 Acinetobacter species were isolated from 2459 samples (20.17%) from the entire hospital, in which Acinetobacter baumannii was 462(93.16%), Acinetobacter lwoffii was 16(3.22%), Acinetobacter junii was 13(2.62%), Acinetobacter haemolyticus was 5(1.00%). Maximum isolates observed from endotracheal tube secretions (39.51%) followed by blood specimens (15.12%), sputum (12.70%), pus swab (8.66%), clean catch (5.84%) and others (18.17%).
Conclusions: In this study, Acinetobacter isolates showed multidrug resistant pattern mostly in inpatients and hence there is a need for emphasizing the importance of hand washing and use of disinfectants in prevention of transmission of infection in health care setups.
Keywords: Acinetobacter; multi-drug resistance; Acinetobacter baumannii; Acinetobacter haemolyticus; VITEK
Full Text
In 1911, a Dutch microbiologist by the name of Martinus Willem Beigerinck discovered an aerobic, gram-negative, non-fermentative bacterium we now know to be of the genus Acinetobacter [1]. Acinetobacter began to be recognized as a significant hospital pathogen in the late 1970s, but at that time it was easily treated as it was susceptible to commonly used antimicrobials. In 1986 a pair of researchers, Bouvet and Grimont, delineated 12 DNA groups of Acinetobacter using DNA-DNA hybridization and proposed 4 new species [2].
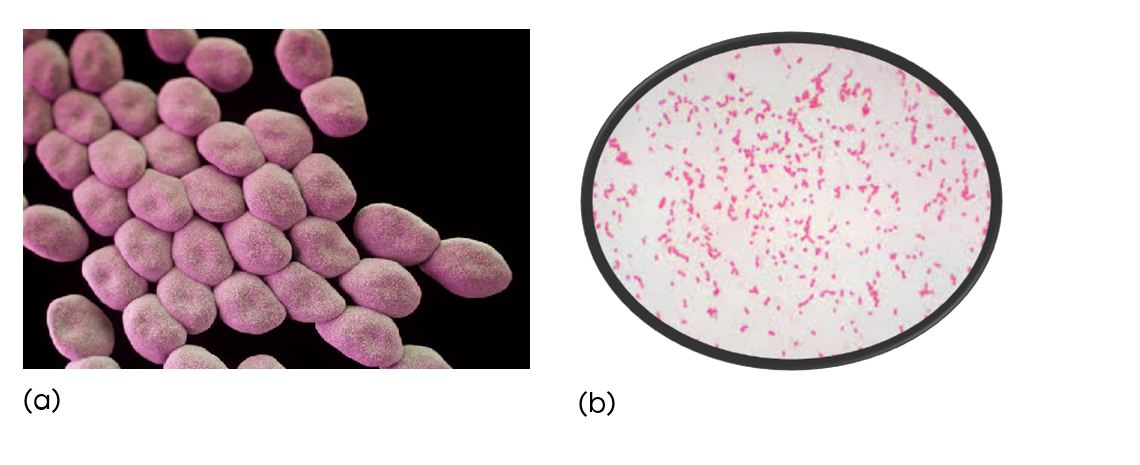
Figure 1: (a) Acinetobacter in electron microscope (b) Acinetobacter in Gram stain.
The genus Acinetobacter are Gram-negative, strictly aerobic, non-fermenting, non-fastidious, non-motile, catalase-positive, and oxidase negative coccobacillary bacteria that can cause healthcare-associated infections and can survive for prolonged periods in the environment and on the hands of healthcare workers [3]. More than two third of Acinetobacter infections are due to Acinetobacter baumannii. Acinetobacter baumannii causes health care associated infections like bacteremia, wound infections, ventilator-associated pneumonia and meningitis [4-7].
Acinetobacter baumannii also has the ability to form biofilms, which may play a role in the process of colonization. Biofilms help the bacteria resist disinfection while also allowing the participating cells to trade resistance genes, further facilitating the persistence of the pathogen [8].
Epidemiology
They can colonise skin, wounds, respiratory and gastrointestinal tract. Acinetobacter spp found in water and soil, also from food (Raw vegetables) and arthropods [9]. Acinetobacter spp broadly found in hospital settings mostly in ICU [10], tropical environment [11], humid climate [11], wars (Recent wars in Kuwait, Iraq and Afghanistan) [4], natural disasters (Earthquake in Marmara Turkey – in 1999) [5]. They can survive in enviroment for weeks. Fomite contamination in hospital promotes transmission. They can survive in dry environment had better survival rates than strains isolated from wet sources [12] and can be found in bed rails due to specific iron acquisition system of Acinetobacter [13].
Widespread environmental contamination is often demonstrated, and outbreaks of infection have been traced to respiratory care equipment, wound care procedures, humidifiers, and patient care items [14-16].
Material and methods
The study is conducted in the department of Microbiology, Krishna Institute of Medical Sciences, Secunderabad. It is a retsopective study in a tertiary care hospital. The study period is from January 2013 to December 2014. The various clinical specimens from inpatients and outpatients were included. The samples were processed as per the standard guidelines. Identification & antibiotic sensitivity testing was done by using GN and AST 281 cards (Vitek 2 compact, BioMerieux) respectively. The quality control for GN card was done by using ATCC 700323– Enterobacter hormaechei, ATCC17666- Stenotrophomonas maltophilia. The quality control for AST N281 was done by ATCC25922 – Escherichia coli, ATCC 27853- Pseudomonas aeruginosa and ATCC 35218- Escherichia coli, are followed as per manufacturer’s instruction. MIC values of antibiotics were obtained and reporting was done as per the CLSI guidelines. The data was captured from the laboratory computer and analysed [17].
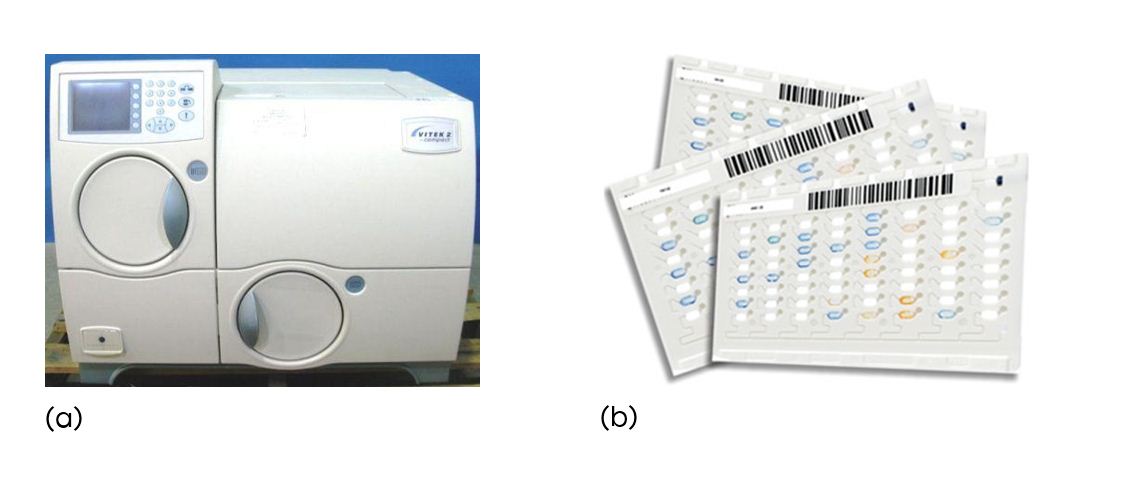
Figure 2: (a) VITEK 2 compact, BioMerieux; (b) GN cards
VITEK 2 compact principle
The VITEK 2 Compact, automated microbiology system utilises growth based technology. It makes use of colorimetric reagent cards that are incubated and interpreted automatically. It has application in clinical laboratories. It has compliance for electronic records and signatures and a colorimetric reagent card to identify GN, GP, YST [9].
The reagent cards have 64 wells that can each contain an individual test substrate. Substrates measure various metabolic activities such as acidification, alkalinization, enzyme hydrolysis, and growth in the presence of inhibitory substances. An optically clear film present on both sides of the card allows for the appropriate level of oxygen transmission while maintaining a sealed vessel that prevents contact with the organism-substrate admixtures. Each card has a pre-inserted transfer tube used for inoculation. Cards have bar codes that contain information on product type, lot number, expiration date, and a unique identifier that can be linked to the sample either before or after loading the card onto the system [18].
Results
A total of 496 Acinetobacter species were isolated from 2459 samples (20.17%) from the entire hospital, in which among inpatients, ICU patients showed maximum isolates (53.69% in 2013; 47.09% in 2014) as compared to ward patients (38.42% in 2013; 45.39% in 2014) (Table1). Acinetobacter baumanii was 462(93.16%), Acinetobacter lwofii was 16 (3.22%), Acinetobacter junii was 13(2.62%), Acinetobacter haemolyticus was 5(1.00%) (Table 2). Maximum isolates observed from endotracheal tube secretions (39.51%) followed by blood specimens (15.12%), sputum (12.70%), pus swab (8.66%), clean catch (5.84%)and others (18.17%). Inpatient showed more isolates (92.11% in 2013 and 92.4% in 2014) than outpatients (7.88%in 2013; 7.5% in 2014) (Table 3). Majority were found to be colistin sensitive (80-90%) followed by gentamycin (50-86%) followed by cefeperazone+sulbactam combination (46-58%) and carbapenems (~50%) as compared to other β-lactam antibiotics (<20%).
Table1:Yearly distribution of Acinetobacter spp isolates (January 2013-december 2014).
|
Total (n=496)
|
|
|
IP
|
OP
|
|
|
ICU
|
WARDS
|
|
YEAR
|
No.
|
%
|
No.
|
%
|
No.
|
%
|
|
2013 (n=203)
|
109
|
53.69%
|
78
|
38.42%
|
16
|
7.88%
|
|
2014 (n=293)
|
138
|
47.09%
|
133
|
45.39%
|
22
|
7.5%
|
Table 2: Different species of Acinetobacter isolates (January 2013-december 2014).
|
Acinetobacter spp.
|
No. of culture positive (496)
|
Culture positive (in %)
(20.17%)
|
|
Acinetobacter baumanni
|
462
|
93.16%
|
|
Acinetobacter lwoffi
|
16
|
3.22%
|
|
Acinetobacter junii
|
13
|
2.62%
|
|
Acinetobacter hemolyticus
|
5
|
1%
|
Table 3: Yearly distribution of Acinetobacter spp. in different clinical specimen.
|
Name of samples
|
2013 (n=203)
|
2014 (n=293)
|
Total (n=496)
|
|
|
No.
|
%
|
No.
|
%
|
|
|
ET secretions
|
66
|
32.5%
|
130
|
44.36%
|
196 (76.87%)
|
|
Blood
|
41
|
20.14%
|
34
|
11.6%
|
75 (31.79%)
|
|
Sputum
|
21
|
10.34%
|
42
|
14.33%
|
63 (24.33%)
|
|
Pus and pus swabs
|
16
|
7.88%
|
27
|
9.21%
|
43 (17.09%)
|
|
Urine (Clean catch)
|
16
|
7.88%
|
13
|
4.43%
|
29 (12.31%)
|
|
Body fluids
|
8
|
3.94%
|
15
|
5.11%
|
23 (9.05%)
|
|
Broncheal wash
|
7
|
3.4%
|
13
|
4.43%
|
20 (7.83%)
|
|
Tissue
|
7
|
3.4%
|
1
|
0.34%
|
8 (3.74%)
|
|
Tracheal secretions
|
7
|
3.4%
|
2
|
0.68%
|
9 (4.08%)
|
|
Urine (Catheter catch)
|
6
|
2.9%
|
6
|
2.04%
|
12 (4.78%)
|
|
Central line
|
5
|
2.4%
|
7
|
2.38%
|
12 (4.78%)
|
|
Wound swabs and fluid
|
2
|
0.98%
|
2
|
0.68%
|
4 (1.66%)
|
|
Catheter tip
|
1
|
0.49%
|
1
|
0.34%
|
2 (0.83%)
|
Discussion
From the study, Acinetobacter is mostly isolated from ET secretions which could be due to its ability to colonise in respiratory tract. They are predominantly found in inpatients, mostly in ICU’s. Acinetobacter has become resistant to a number of antimicrobials due to its overuse. Acinetobacter has acquired an impressive intrinsic resistance mechanisms and can acquire new mechanisms via plasmids, integrons, and transposons. It also acquire resistance through change in porins and efflux pump.
Despite the various mechanisms of resistance Acinetobacter are susceptible to few antimicrobials including - colistin, gentamycin, cefperazone+sulbactam combination and carbapenems (i.e. imipenem and meropenem).
Conclusions
In this study, Acinetobacter isolates showed multi-drug resistant pattern mostly in inpatients. Measures to prevent the intrahospital transmission of Acinetobacter is done by monitoring by surveillance of multi-drug resistant Gram negative bacilli hospital acquired infection. Success in infection control has been attained by if health care workers are educated about the proper way to manage MDR Acinetobacter and emphasizing the importance of hand washing and use of disinfectants in prevention of transmission of infection in health care setups.
Acknowledgements
I would like to acknowledge the Department of Microbiology, KIMS Hospital, Secunderabad, for giving the opportunity to carry out the study.
Conflict of interest
]Authors declare no conflict of interest.
References
1. Nemec A, Musílek M, Maixnerová M, De Baere T, van der Reijden TJ, et al. Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. Int J Syst Evol Microbiol. 2009; 59:118–124.
2. Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991; 29(2):277–282.
3. Young LS, Sabel AL, Price CS. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2007; 28(11):1247–1254.
4. Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005; 11(8):1218–1224.
5. Oncül O, Keskin O, Acar HV, Küçükardali Y, Evrenkaya R, et al. Hospital-acquired infections following the 1999 Marmara earthquake. J Hosp Infect. 2002; 51(1):47–51.
6. Young LS, Sabel AL, Price CS. Epidemiologic, clinical, and economic evaluation of an outbreak of clonal multidrug-resistant Acinetobacter baumannii infection in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2007; 28(11):1247–1254.
7. Van Looveren M, Goossens H, ARPAC Steering Group. Antimicrobial resistance of Acinetobacter spp. in Europe.Clin Microbiol Infect. 2004; 10(8):684–704.
8. Rajamohan G, Srinivasan VB, Gebreyes WA. Biocide-tolerant multidrug-resistant Acinetobacter baumannii clinical strains are associated with higher biofilm formation. J Hosp Infect. 2009; 73(3):287–289.
9. Houang ET, Chu YW, Leung CM, Chu KY, Berlau J, et al. Epidemiology and infection control implications of Acinetobacter spp. in Hong Kong. J Clin Microbiol. 2001; 39(1):228–234.
10. Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005; 11(11):868–873.
11. Anstey NM, Currie BJ, Hassell M, Palmer D, Dwyer B, et al. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J Clin Microbiol. 2002; 40(4):685–686.
12. Houang ET, Sormunen RT, Lai L, Chan CY, Leong AS. Effect of dessication on the structural appearance of Acinetobacter baumannii and Acinetobacter lwoffii. J Clin Pathol. 1998; 51(10):786–788.
13. Catalano M, Quelle LS, Jeric PE, Di Martino A, Maimone SM. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J Hosp Infect. 1999; 42(1):27–35.
14. Podnos YD, Cinat ME, Wilson SE, Cooke J, Gornick W, et al. Eradication of multi-drug resistant Acinetobacter from an intensive care unit. Surg Infect (Larchmt) 2001; 2(4):297–301.
15. Aygun G, Demirkiran O, Utku T, Mete B, Urkmez S, et al. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect. 2002; 52(4):259–262.
16. Zanetti G, Blanc DS, Federli I, Raffoul W, Petignat C, et al. Importation of Acinetobacter baumanniiinto a burn unit: a recurrent outbreak of infection associated with widespread environmental contamination. Infect Control Hosp Epidemiol. 2007; 28(6):723–725.
17. Jean, Franklin, Patrica, Geaorge, Janet, et al. M100-S25 performance standards for antimicrobial susceptibility testing. Clinical laboratory standards institute guidelines 2014; 34:120–122.
18. David H Pincus. Microbial Identification using Biomeuriux Vitek 2 system. p2–3.