Full Text
Case report
A 40-year-old female, non-diabetic, non-hypertensive, presented with history of persistent vomitings since 6 months, 3-5 episodes of non-projectile, bilious vomitings per day, vomitus contains food particles and vomitings not related to food intake. History of constipation and increased frequency of urination present. History of significant weight loss present (lost 15kgs in 6 months). History of mild to moderate, non-progressive headache present since 3years but no history of visual disturbances. No history of odynophagia/ dysphagia/ dyspepsia/ cough/ haematemesis/ melena/ pain abdomen/ diarrhoea/ fever/ anorexia/ drug abuse/ native medicine abuse. Patient was treated outside with proton pump inhibitors, antacids and prokinetics. On examination, she was emaciated, dehydrated and very pale. Her blood pressure was 110/80 mmHg, and pulse rate was 88/min and regular. Cardiovascular, respiratory, and abdominal examinations were unremarkable. Laboratory work up revealed normal blood count except for Hb 9.9g/dl (microcytic, hypochromic), random plasma glucose - 126 mg/dL (80-140), serum creatinine – 1.5 mg/dL (0.5-1.4), sodium - 140 mmol/L (135-145), potassium - 3.5 mmol/L (3.5-5), chloride 102 mmol/L (90-106), bicarbonate - 28 mmol/L (22-29), magnesium – 2.2 mg/dL (1.7-2.2), serum bilirubin -1.1 mg/dl (0.2-1.0), aspartate transaminase - 26 U/L (<40), alanine aminotransferase - 20 U/L (<40), alkaline phosphatase 120 IU/L (<110), albumin - 3.7 g/dL (3.5-5). Total cholesterol 220mg/dl, LDL-142 mg/dl, triglycerides- 126mg/dl. Ultrasound abdomen was unremarkable except mild hepatomegaly. Upper gastrointestinal endoscopy was normal and acid peptic disease was ruled out. Serum cortisol (8 am) - 18 μ/dL (9-25) was normal and excluded adrenal insufficiency. Thyroid profile was normal and excluded thyrotoxicosis {TSH 0.46 μIU/ml (0.3-4.5), total thyroxine (T 4) 10.2 μg/dL (4.5-12.5)}. Serum amylase was normal and excluded pancreatitis. Since patient had increased frequency of urination, 24-hour intake/ output chart was maintained which showed intake of 3L and urine output of 3.6L. To confirm diabetes insipidus, urine specific gravity and urine osmolality was advised which revealed urine specific gravity 1.010 and urine osmolality of 186 mOsm/kg of water (300-900). Since patient had headache, vomitings and diabetes insipidus, MRI brain was advised to rule out posterior pituitary lesion. MRI brain showed absence of T1 hyperintensity in posterior pituitary. Further work up for diabetes insipidus includes serum ADH which was 5 pg/ml (1-5 pg/ml). Since serum creatinine was elevated, ABG was done to rule out renal tubular acidosis. Corrected serum calcium was 15 mg/dl (8.4-10.4 mg/dl). 24-hour urine for calcium was 800 mg (<300). Electrocardiogram showed slightly shortened QTc interval (421 msec).
Echocardiogram was normal. Vomitings and nephrogenic diabetes insipidus, secondary to hypercalcemia were confirmed. Since patient has anaemia, hypercalcemia and renal insufficiency, in differential diagnosis multiple myeloma, sarcoidosis, and hyperparathyroidism were considered. Work up revealed no lytic lesions in x-ray skull, serum 25-hydroxy vitamin D-19 ng/ml (30-100), serum phosphate 2.5mg/dl (2.5-4.5) and serum intact parathyroid hormone levels 1800 pg/ml (8-50). Work up for primary hyperparathyroidism revealed probable right superior parathyroid adenoma in ultrasonography of neck (Figure 1), which was confirmed with Sestamibi nuclear scan (Figure 2).
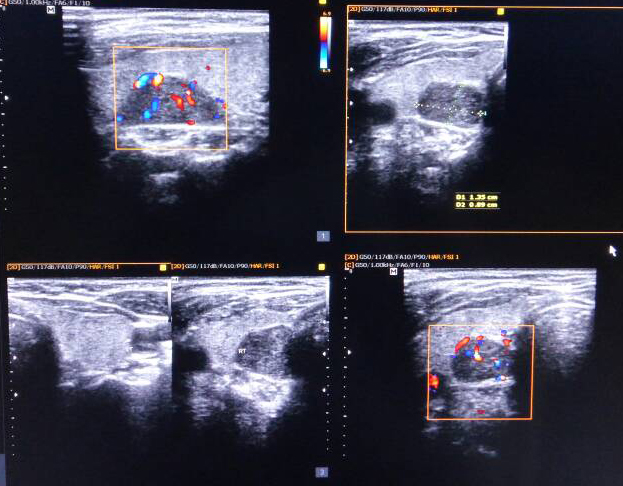
Figure 1: Ultrasound neck showing enlarged right parathyroid gland.
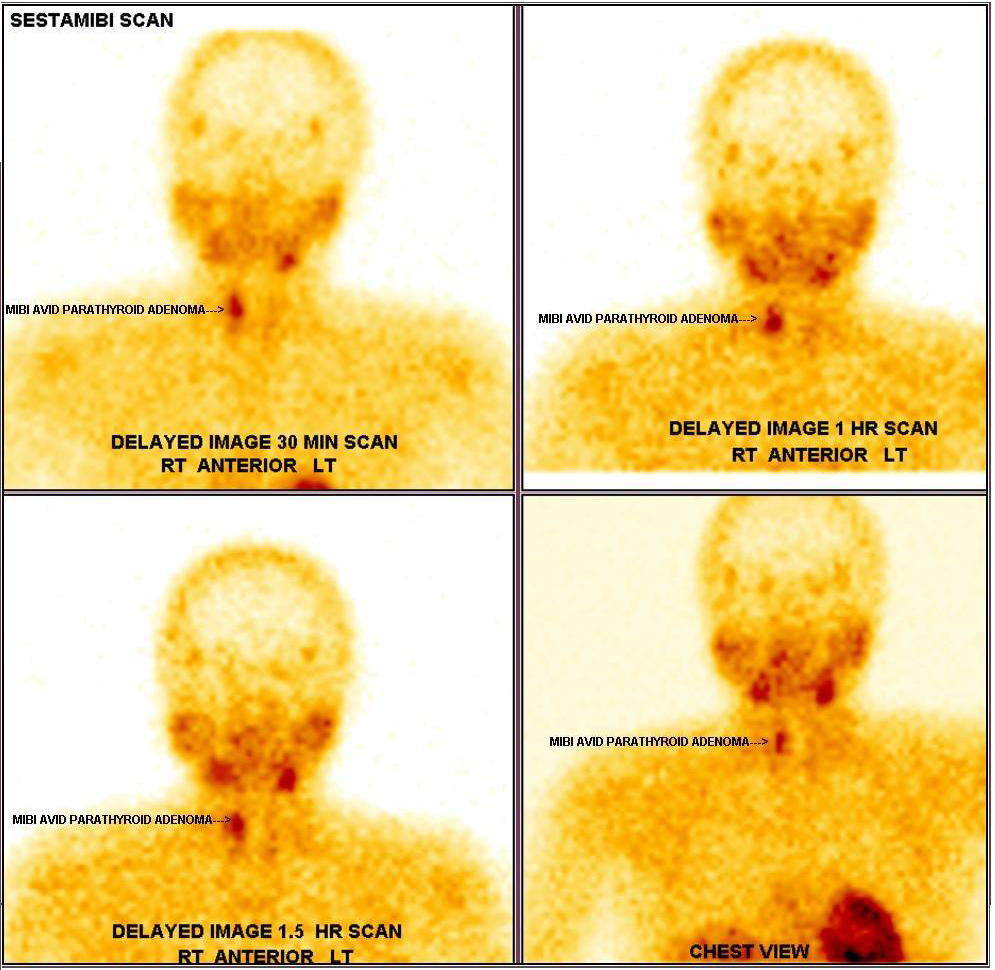
Figure 2: Sestamibi scan showing right parathyroid adenoma.
Bone densitometry showed T scores at lumbar spine of -1.2 and at the total hip of -1.4. Patient was treated with intravenous crystalloids, steroids, and calcitonin prior to surgery.
Pre-operative serum calcium was 14 mg/dl. Right superior parathyroid adenectomy was done by general surgeon and post-operatively after 15 minutes, repeat serum intact PTH was advised and it was normal (56 pg/ml). Post-operative patient had hoarseness of voice secondary to injury to right recurrant laryngeal nerve. Post-operative serum calcium was 11.7 mg/dl, 9.6 mg/dl, 9.1mg/dl on day 1,2,3 respectively. On post-operative Day 3, patient had paraesthesias all over the body and repeat serum calcium was advised (8.1mg/dl). It was treated with injection calcium gluconate and oral supplementation with pills containing calcium carbonate 500mg plus Calcitriol 0.25 mg thrice daily was advised. From POD 4 to POD 10, her serum calcium was between 7.1-7.8 mg/dl despite of increasing number of calcium pills to 8 pills per day. Hungry bone syndrome was suspected and serum magnesium, serum phosphate and serum potassium were estimated. Serum magnesium was decreased 1.4 mg/dl, serum phosphate 2.1 mg/dl and serum potassium increased 5.7meq/l (serum creatinine- 1.3 mg/dl). Hypomagnesemia was corrected with magnesium sulphate and hyperkalemia was treated with potassium binding resins and salbutamol nebulization. With 4gm of calcium carbonate and calcitriol 2 µg on POD14, her calcium level was 8.0 mg/dl. Patient was discharged with oral calcium pills 3-3-2 per day (calcium carbonate 500mg, calcitriol 0.25mg) and potassium binding resins. Histopathology of excised parathyroid gland was suggestive of benign parathyroid adenoma. Genetic screening for MEN1 & 2 gene mutation was advised.
Discussion
Primary hyperparathyroidism is characterized by inappropriate, autonomous secretion of parathyroid hormone (PTH), mostly due to parathyroid adenoma and rarely due to hyperplasia or carcinoma. Most common cause of hypercalcemia is due to primary hyperparathyroidism [1]. The incidence of primary hyperparathyroidism increases with age with peak in seventh decade, common in elderly women while it is more or less equal in men and women before 45 years age [2].
Asymptomatic hyperparathyroidism is prevalent in developed countries because of increase in biochemical screening, contrary to this symptomatic hyperparathyroidism (nephrolithiasis, bone pains, fracture) is more common in India [3, 4]. The clinical presentation of primary hyperparathyroidism in India as per systematic review of Pradeep et al. [4], is bone disease (77%), proximal muscle weakness (54%), brown tumours (42%), fractures (40%), renal disease (36%), psychiatric symptoms (26%), pancreatitis (15%), and asymptomatic (5.6%). In India, gastrointestinal presentations of hyperparathyroidism such as anorexia, nausea, vomiting, constipation and pain abdomen were more prevalent in the last decade [5]. Viral N et al. [5] found in their study that presentation with nausea, vomiting (39%), constipation (48%), and polyuria (28%) in the last decade. Our patient even though presented with nausea, vomitings, she also had constipation and polyuria. Hyperparathyroidism usually causes weight gain, in contrast weight loss in our patient was because of dehydration due to persistent vomitings daily for 6 months.
Definitive treatment of primary hyperparathyroidism is parathyroidectomy. Patients with mild hypercalcemia (<12 mg/dl) can be directly posted for surgery while correction of calcium before surgery is preferred in case of moderate to severe hypercalcemia to avoid complications. Postoperative hypocalcaemia is common, following parathyroidectomy, which usually starts on POD3.
Hungry bone syndrome (HBS) was noticed in 24% to 82% of the cases postoperatively [3, 6].
HBS refers to the profound (serum calcium <8.4 mg/dl) and prolonged (longer than 4th day post-operatively) hypocalcaemia, which follows parathyroidectomy. This is usually associated with skeletal manifestations in majority of cases, however this was reported in less than 6% of cases without radiological evidence of PTH related bone disease. The risk factors for hungry bone syndrome in our case were due to very high preoperative parathyroid hormone levels (1800 pg/ml), coexistent vitamin d deficiency (19 ng/ml), and high preoperative serum calcium levels (14 mg/dl). Treatment of HBS is replenishing the calcium deficit by using high doses of calcium which varies between 6 and 12 g/day [7] and supplementation of vitamin D. Correction of magnesium deficiency is required for resolution of the hypocalcaemia. Calcium levels may return to normal after few weeks to months in HBS.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Bilezikian JP, Cusano NE, Khan AA, Liu JM, Marcocci C. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016; 2:16033.
[2] Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: An update on the changing epidemiology of the disease. J Bone Miner Res. 2006; 21(1):171–177.
[3] Bhansali A, Masoodi SR, Reddy KS, Behra A, Das Radotra B, et al. primary hyperparathyroidism in north India: A description of 52 cases. Ann Saudi Med. 2005; 25(1):29–35.
[4] Pradeep PV, Jayashree B, Mishra A, Mishra SK. Systematic review of primary hyperparathyroidism in India: The past, present, and the future trends. Int J Endocrinol. 2011; 2011:921814.
[5] Shah VN, Bhadada S, Bhansali A, Behera A, Mittal BR. Changes in clinical & biochemical presentations of primary hyperparathyroidism in India over a period of 20 years. Indian J Med Res. 2014; 139(5):694–699.
[6] Pradeep PV, Mishra A, Agarwal G, Agarwal A, Verma AK, et al. Long-term outcome after parathyroidectomy in patients with advanced primary hyperparathyroidism and associated vitamin D deficiency. World J Surg. 2008; 32(5):829–835.
[7] Rathi MS, Ajjan R, Orme SM. A case of parathyroid carcinoma with severe hungry bone syndrome and review of literature. Exp Clin Endocrinol Diabetes. 2008; 116(8):487–490.