Original Research
2021
March
Volume : 9
Issue : 1
A retrospective study of antimicrobial susceptibility pattern of aerobic microbial isolates from urine samples of patients attending a tertiary care hospital
Ahmad S, Jahan N, Ahmad SS, Singh N, Khatoon R
Pdf Page Numbers :- 5-10
Siraj Ahmad1, Noor Jahan2, Syed Saif Ahmad3, Nidhi Singh2,* and Razia Khatoon4
1Department of Community Medicine, Integral Institute of Medical Sciences & Research, Integral University, Lucknow- 226026, UP, India
2Department of Microbiology, Integral Institute of Medical Sciences and Research, Lucknow-226026, UP, India
3Charak Diagnostics Centre, Chowk, Lucknow-226003, UP, India
4Department of Microbiology, Hind Institute of Medical Sciences, Mau, Ataria, Sitapur-261303, UP, India
*Corresponding author: Miss. Nidhi Singh, Department of Microbiology, Integral Institute of Medical Sciences & Research, Integral University, Lucknow-226026, U.P., India. Email: Singhnidhi892@yahoo.com
Received 9 September 2020; Revised 25 November 2020; Accepted 8 December 2020; Published 22 December 2020
Citation: Ahmad S, Jahan N, Ahmad SS, Singh N, Khatoon R. A retrospective study of antimicrobial susceptibility pattern of aerobic microbial isolates from urine samples of patients attending a tertiary care hospital. J Med Sci Res. 2021; 9(1):5-10. DOI: http://dx.doi.org/10.17727/JMSR.2021/9-2
Copyright: © 2021 Ahmad S et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Urinary tract infections (UTIs) are one of the most common infections encountered in clinical practice.
Objective: This is a retrospective study to evaluate the antimicrobial susceptibility pattern of aerobic microbial isolates from urine samples of patients with complaints suggestive of UTI.
Materials and methods: A total of 3116 urine samples which were received in the Department of Microbiology during the study period from April 2018 to March 2019 were analyzed.
Results: Out of 3116 urine samples from both outpatient (OPD) and inpatient department (IPD), 2614 samples showed either growth of contaminants or insignificant or no growth of microorganisms, whereas, 502 samples showed significant microbial growth on aerobic culture giving overall prevalence of UTI in the study population to be 16.1%. Of these 502 culture positives, majority was found to be from OPD (51.0%) patients, amongst females (62.9%), and Escherichia coli being the commonest isolate (49.8%). The antibiotic sensitivity of uroisolates of Escherichia coli were found to range from 46.0-70.0%.
Conclusion: In view of the increasing drug resistance amongst pathogens causing UTI, antimicrobial susceptibility should be done before initiating definitive therapy. These data may be used to formulate local antibiotic policies in order to assist clinicians in the rationale use of antibiotics.
Keywords: aerobic microbial isolates; urinary tract infection; antimicrobial susceptibility test; retrospective analysis.
Full Text
Introduction
A urinary tract infection (UTI) is an infection in any part of urinary tract comprising of kidneys, ureters, bladder and urethra. The lower urinary tract i.e. the bladder and the urethra are often involved. UTIs are caused by bacteria, fungi and rarely by viruses. Females suffer from UTI more often than the males because of the shortness of urethra and its anal proximity. Risk factors include immune suppression, trauma, foreign body, broad spectrum antibiotic use, infused body fluids like saline irrigations and also urinary catheterization. Escherichia coli from the gut is the cause of 80 – 85% UTIs, followed by Klebsiella, Proteus, Pseudomonas and Citrobacter [1, 2]. The presence of gram positive organisms like Staphylococcus aureus and Enterococcus has also increased [2].
It is estimated that annually, worldwide around 7 million and 1 million patients with UTI attend the outpatient and emergency department respectively. Whereas, 100,000 hospitalizations occur annually due to UTI [3]. Most of the times these UTIs are treated empirically without any antibiotic susceptibility testing which leads to increased drug resistance in bacteria against commonly used antibiotics [4].
The manifestations of UTI may vary from mild asymptomatic cystitis to pyelonephritis and septicemia [5]. Untreated UTI can result in serious complications such as recurrent infection, pyelonephritis with sepsis, pre-term birth in pregnant females, and renal damage in young children. Additionally, complexities brought on by inappropriate antimicrobial use could result in high rate of antimicrobial resistance [6].
Recently, several studies have revealed increasing trends of antibiotic resistance [7]. The antibiotic susceptibility pattern of uropathogens may vary according to the type of healthcare provided (primary or tertiary care at hospitals or other healthcare settings), different environments and geographical location. Periodic evaluation of such pattern is necessary to update this information [8, 9].
Hence, keeping the above facts in mind, the present study was carried out at a tertiary care hospital to evaluate the spectrum of aerobic microbial isolates responsible for UTI and their resistance pattern against the commonly used antibiotics.
Materials and methods
A retrospective study was done over a period of one year from March 2018 to April 2019 among all patients clinically suspected of having UTI and attending outpatient department as well as those admitted in wards of Integral Institute of Medical Sciences and Research, Lucknow, India, whose urine samples were received in the Department of Microbiology were analyzed. The present study was approved by Institutional Ethical Committee letter number IEC/IIMS&R/2020/17 conducted on 27th January 2020.
Most of the urine samples were mid-stream clean catch, especially for the outpatient group and for many inpatients. The pathogen(s) grown from the first sample of urine were considered in the analysis. Repeated samples (from patients who were already included), samples that grew more than two types of organism, or had evidence of perineal contamination were not included for analysis.
A total of 3116 urine samples were processed for determining colony count on cysteine lactose electrolyte-deficient (CLED) agar medium using calibrated loops, as per standard protocol [10]. Samples showing growth of Gram negative organisms with colony count >105 colony forming units (CFU/ml) with single morphotype or up to 2 types, were considered significant and processed further for identification and susceptibility testing. Gram positive organisms were processed, if isolated as pure growth even when the colony counts were <104 CFU/ml.
Antimicrobial susceptibility testing was done by Kirby-Bauer disk diffusion method and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines 2018. All antibiotic discs were procured from HIMEDIA (Mumbai, India). Quality control of media and discs were performed using American Type Culture Collection (ATCC) control strains [10, 11].
Statistical analysis
The collected data was analyzed using SPSS data editor software, Chicago, version 20. Percentage of variables was calculated.
Results
A total of 3116 urine samples from outdoor and indoor patients were processed, out of which 2614 showed either growth of contaminants or insignificant or no growth of microorganisms, whereas, 502 samples showed significant microbial growth on aerobic culture. Overall prevalence of UTI in the study population was about 16.1%.
In our study it was found that out of 502 culture positives, majority belonged to OPD (N=256) patients as compared to IPD (N=246) patients, as shown in Figure 1.
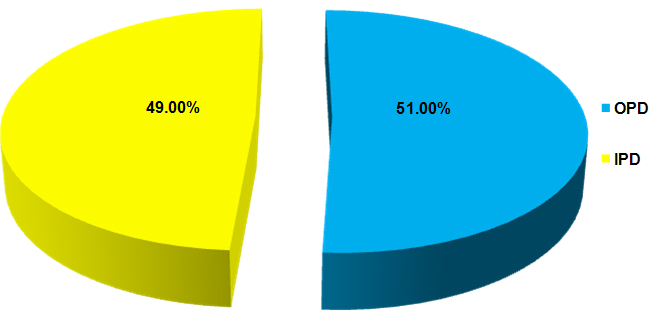
Figure 1: Distribution of culture positive patients according to their registration status (N=502).
As shown in Figure 2, UTI was found to be more prevalent amongst female patients (N=316) as compared to male patients (N=186).
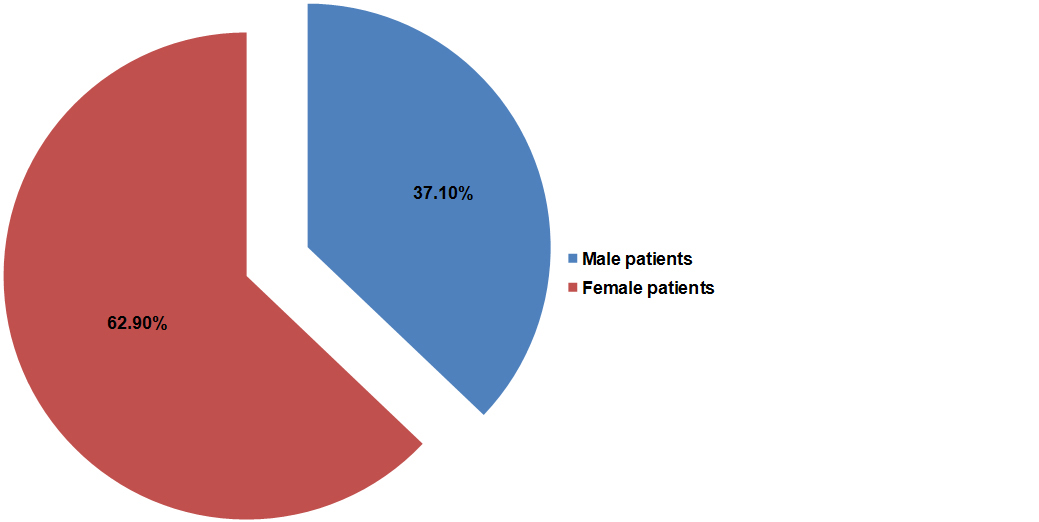
Figure 2: Distribution of culture positive patients according to their gender (N=502).
It was observed in our study that majority of culture positive patients belonged to age group 20-29 years (28.3%), followed by age group 30-39 years (21.9%), 40-49 years (12.3%), 50-59 years (9.9%), 60-69 years (7.6%), 10-19 years (7.6%), 0-9 years (7.2%), 70-79 years (4.4%) and least among age groups 80-89 years and 90-99 years (0.4% each) as depicted in Figure 3.
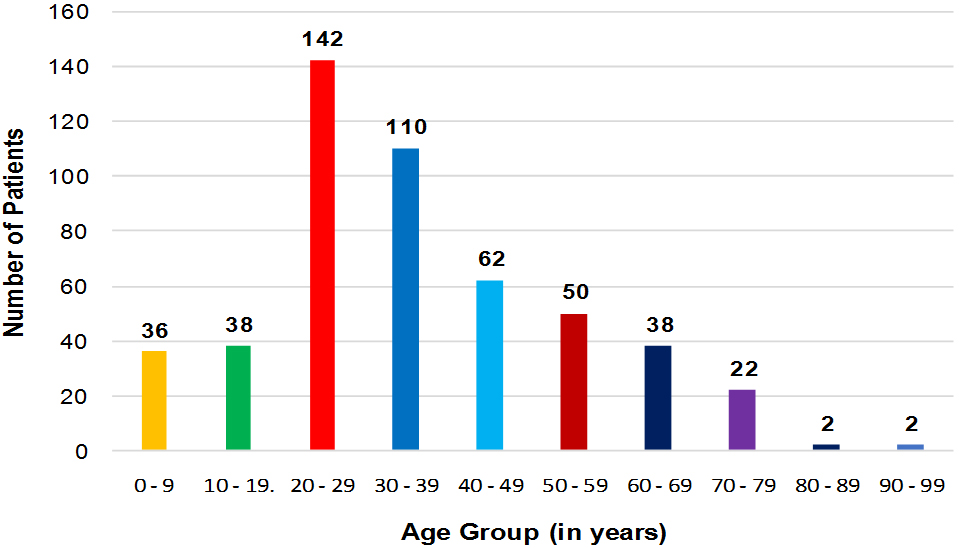
Figure 3: Distribution of culture positive patients according to their age group (N=502).
As shown in Figure 4, out of 502 culture positive samples that yielded significant microbial growth, Escherichia coli was the commonest Gram negative isolate (49.8%), followed by Klebsiella spp. (15.9%), Acinetobacter spp. (4.0%), Pseudomonas spp. (2.2%), Citrobacter spp. (1.2%), Proteus spp. (0.8%) and Morganella spp. (0.8%). Among the Gram positive organisms commonest bacteria isolated was Enterococcus spp. (14.7%) followed by Staphylococcus aureus (6.0%), coagulase negative Staphylococci (2.0%) and Streptococcus agalactiae (1.0%). Candida spp. constituted 1.6% of the total isolates.
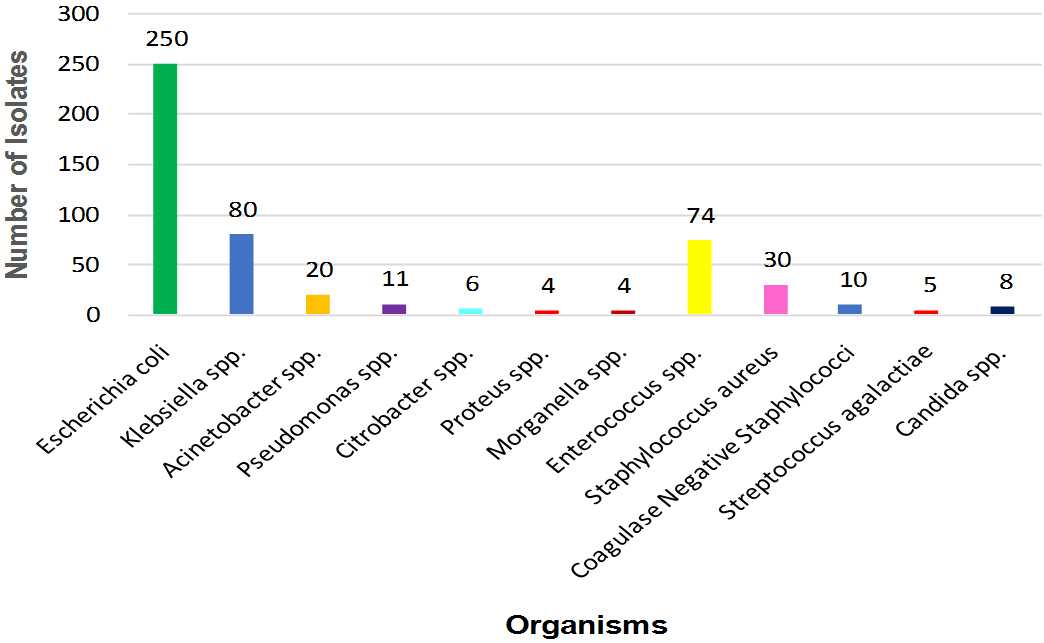
Figure 4: Organism wise distribution of culture positive isolates (N=502).
The antimicrobial susceptibility pattern of the common isolates i.e. Escherichia coli, Klebsiella spp., Enterococcus spp., and Staphylococcus aureus were further studied. Table 1 shows the susceptibility pattern of urinary isolates of Escherichia coli. It was found that isolates of Escherichia coli showed highest sensitivity towards nitrofurantoin (70.0%), followed by piperacillin/tazobactam (65.6%). It was least susceptible to cotrimoxazole (46.0%) and meropenem (48.0 %).
Table 1: Antibiotic susceptibility pattern of urinary isolates of Escherichia coli for the commonly used antibiotics (N = 250).
|
Antibiotics tested
|
Number of sensitive isolates (%)
|
Number of resistant isolates (%)
|
|
Amikacin
|
153(61.2%)
|
97(38.8%)
|
|
Imipenem
|
125(50.0%)
|
125(50.0%)
|
|
Nitrofurnatoin
|
175(70.0%)
|
75(30.0%)
|
|
Ampicillin/sulbactam
|
129(51.6%)
|
121(48.4%)
|
|
Piperacillin/tazobactam
|
164(65.6%)
|
86(34.4%)
|
|
Gentamicin
|
125(50.0%)
|
125(50.0%)
|
|
Meropenem
|
120(48.0%)
|
130(52.0%)
|
|
Cotrimoxazole
|
115(46.0%)
|
135(54.0%)
|
Abbreviations: N = Number of isolates.
The uroisolates of Klebsiella spp. were found to be highly sensitivity tomeropenem (82.5%), followed by imipenem (76.3%). However, they showed least sensitivity for nitrofurantoin (50.0%), followed by cotrimoxazole (55.0%) as shown in Table 2.
Table 2: Antibiotic susceptibility pattern of urinary isolates of Klebsiella spp. for the commonly used antibiotics (N = 80).
|
Antibiotics tested
|
Number of sensitive isolates (%)
|
Number of resistant isolates (%)
|
|
Amikacin
|
60(75.0%)
|
20(25.0%)
|
|
Imipenem
|
61(76.3%)
|
19(23.7%)
|
|
Nitrofurnatoin
|
40(50.0%)
|
40(50.0%)
|
|
Gentamicin
|
52(65.0%)
|
28(35.0%)
|
|
Meropenem
|
66(82.5%)
|
14(17.5%)
|
|
Cotrimoxazole
|
44(55.0%)
|
36(45.0%)
|
|
Ciprofloxacin
|
45(56.3%)
|
35(43.7%)
|
Abbreviations: N = Number of isolates.
As depicted in Table 3 the urinary isolates of Enterococcus spp. showed highest sensitivity of 100% for high level gentamicin, high level streptomycin and vancomycin, while they showed least sensitivity for ciprofloxacin (47.3%).
Table 3: Antibiotic susceptibility pattern of urinary isolates of Enterococcus spp. for the commonly used antibiotics (N = 74).
|
Antibiotics tested
|
Number of sensitive isolates (%)
|
Number of resistant isolates (%)
|
|
High level gentamicin
|
74(100%)
|
0(0%)
|
|
High level streptomycin
|
74(100%)
|
0(0%)
|
|
Vancomycin
|
74(100%)
|
0(0%)
|
|
Linezolid
|
65(87.8%)
|
9(12.2%)
|
|
Nitrofurantoin
|
55(74.3%)
|
19(25.7%)
|
|
Ciprofloxacin
|
35(47.3%)
|
39(52.7%)
|
|
Teicoplanin
|
68(91.9%)
|
6(8.1%)
|
Abbreviations: N = Number of isolates.
Table 4 shows the antimicrobial susceptibility pattern of uroisolates of staphylococcus aureus. These isolates were found to have highest sensitivity of 100% to vancomycin, linezolid, tobramycin and teicoplanin, followed by sensitivity to nitrofurantoin (80.0%) and least sensitivity for ciprofloxacin (46.7%) followed by cefoxitin (50%).
Table 4: Antibiotic susceptibility pattern of urinary isolates of Staphylococcus aureus for the commonly used antibiotics (N = 30).
|
Antibiotics tested
|
Number of sensitive isolates (%)
|
Number of resistant isolates (%)
|
|
Vancomycin
|
30(100%)
|
0(0%)
|
|
Linezolid
|
30(100%)
|
0(0%)
|
|
Ciprofloxacin
|
14(46.7%)
|
16(53.3%)
|
|
Cefoxitin
|
15(50%)
|
15(50%)
|
|
Nitrofurantoin
|
24(80.0%)
|
6(20.0%)
|
|
Tobramycin
|
30(100%)
|
0(0%)
|
|
Teicoplanin
|
30(100%)
|
0(0%)
|
|
Cotrimoxazole
|
23(76.7%)
|
7(23.3%)
|
Abbreviations: N = Number of isolates.
Discussion
UTI accounts for huge burden on health care systems due to its high prevalence in both community and nosocomial settings. UTI is caused by variety of pathogens including Escherichia coli, Klebsiella spp, Proteus spp, Staphylococcus aureus, coagulase negative Staphylococci and also Candida spp. [12]. The prevalence and antimicrobial susceptibility of uropathogens may vary with time and geographical area, and therefore continuous surveillance of antibiotic susceptibility patterns of urinary pathogens at local level is crucial in dealing with emerging problems of antibiotic resistance and provide assistance in managing with effective initial therapy [13, 14].
The present retrospective study highlights the distribution of UTI causing organisms and the antibiotic resistance patterns of the common isolated uropathogens in the population seeking healthcare services from a tertiary care hospital at Lucknow.
Overall prevalence of UTI in the study population was about 16.1%. We found a greater prevalence of UTI in female patients (62.9%) as compared to male patients (37.1%) which is in concordance with the finding of another study done in Meerut which reported that out of 155 culture positives higher prevalence of UTI was seen among female patients (103/155) as compared to male patients (52/155) [15]. Females are more prone to develop UTI, probably due to the characteristic anatomy of the urethra and the effect of normal physiological changes that affect the urinary tract – short urethra, its proximity to the anus, urethral trauma during intercourse, dilation of the urethra and stasis of urine during pregnancy [16-18].
Escherichia coli were the most frequently encountered uropathogen in our study, followed by Klebsiella spp. and Enterococcus spp. The isolation rate of urinary pathogens is consistent with reports of other recently published studies [19-23]. However, studies from some other parts of the country have shown different isolation rates, probably due to variation in sample size, geographical location or population.
The higher prevalence of UTI in the present study was found in sexually active young patients between the age group 20 – 39 years. Similarly a study done in Gujarat reported higher prevalence of UTI among patients between age group of 31-45 years (44.8%) [24].
Antibiotic resistance has become a major clinical problem worldwide and has increased over the years. Most of the isolates were resistant to multiple antibiotics at our setting. High level of resistance to Ampicillin/Sulbactam was seen amongst the most commonly isolated uropathogen i.e. Escherichia coli. Fluoroquinolones, which are the mainstay for treatment of urinary tract infections, were not found to be useful even among Gram negative bacilli due to their reduced sensitivity. This is similar to previous studies in India [19].
Klebsiella spp. have the ability to acquire resistance genes by mutations and more commonly by transmissible plasmids. Progressive spread and increasing incidence of carbapenem resistance among Klebsiella spp. has become a severe public health issue [25]. In our study Klebsiella spp. showed reduced sensitivity of 76.3% to imepenem and 82.5% sensitivity to meropenem. Since carbapenems are often the last line of defense against resistant Gram-negative infections, resistance to these antibiotics could result in greater morbidity, mortality, costs, and prolonged hospital stay [25].
As far as gram positive cocci are concerned vancomycin and linezolid were the most effective antibiotics with 100% sensitivity as reported in various studies [26-28]. Very high resistance was seen to ciprofloxacin amongst Enterococcal isolates which is in agreement with other studies [26, 27].
The Infectious Diseases Society of America (IDSA) guidelines consider nitrofurantoin and co- trimoxazole as current standard therapy for uncomplicated UTI in women. However, the guidelines specify that local antimicrobial susceptibility patterns should be taken into account [29]. In our study reduced sensitivity for both nitrofurantoin and co-trimoxazole was detected amongst the commonly isolated Gram negative bacilli (Escherichia coli) and Gram positive cocci (Enterococcus spp.).
Conclusion
To conclude, antibiotic resistance has become a huge public health problem as it leads to limited treatment options, increased treatment costs and hospital stay. Our study reported that the isolated uropathogens showed high levels of resistance to multiple urinary antimicrobial agents. Therefore, it is mandatory to routinely test the antimicrobial susceptibility pattern of the isolated microorganisms. This is of utmost importance to prepare the antibiotic policy of the hospital and thereby help the clinicians to give empirical treatment of UTI.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Kalpana S, Hegadi SS, Ramesh K. Characterization and antimicrobial susceptibility testing of uropathogens from urinary tract infections. Int J Curr Microbiol Appl Sci. 2015; 4(2):1010–1016.
[2] Ghadage DP, Muley VA, Sharma J, Bhore AV. Bacteriological profile and antibiogram of Urinary Tract Infections at a tertiary care hospital. Natl J Lab Med. 2016; 5(4):20–24.
[3] Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004; 38(8):1150–1158.
[4] Chiu C. Definitions, classifications, and antibiotics. In: Ran´e A, Dasgupta R, editors. Urinary tract infection: Clinical perspectives on urinary tract infection. London: Springer-Verlag, 2013; pp.1–10.
[5] Naveen R, Mathai E. Some virulence characteristics of uropathogenic Escherichia coli in different patient groups. Indian J Med Res. 2005; 122(2):143–147.
[6] Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015; 13(5):269–284.
[7] Nickel JC. Urinary tract infections and resistant bacteria: Highlights of a symposium at the combined meeting of the 25th International congress of chemotherapy (ICC) and the 17th European congress of clinical microbiology and infectious diseases (ECCMID), March 31-April 3, 2007, Munich, Germany. Rev Urol. 2007; 9(2):78–80.
[8] Rudramurthy KG, Kumaran R, Geetha RK. Etiology and antimicrobial susceptibility pattern of bacterial agents from urinary tract infection in a tertiary care centre. Int J Sci Stud. 2015; 2(11):125–127.
[9] Gupta V, Yadav A, Joshi RM. Antibiotic resistance pattern in uropathogens. Indian J Med Microbiol. 2002; 20(2):96–98.
[10] Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A. Laboratory strategy in the diagnosis of infective syndromes. In: Mackie and McCartney practical medical microbiology. Collee JG, Fraser AG, Marmion BP, Simmons A, editors. 14th ed. Edinburgh, Churchill Livingstone, 2006; pp.84–90.
[11] Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplements M100. 950 West Valley Road, Suite 2500, Wayne, Pennsylvania- 19087, USA, 2018.
[12] Bano S, Tunio SA, Memon AA, Detho H, Bano R, et al. Evaluation of antibiotic susceptibility patterns of uropathogens circulating in Hyderabad, Pakistan. Khyber Med Univ J. 2014; 6(3):110–115.
[13] Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010; 7(12):653–660.
[14] Banerjee S. The study of urinary tract infections and antibiogram of uropathogens in and around Ahmadnagar, Maharashtra. The Internet J Infect Dis. 2009; 9(1):1–5.
[15] Prakash D, Saxena RS. Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of meerut city, India. ISRN Microbiol. 2013; 2013(749629):1–13.
[16] Minardi D, d'Anzeo G, Cantoro D, Conti A, Muzzonigro G. Urinary tract infections in women: Etiology and treatment options. Int J Gen Med. 2011; 4:333–343.
[17] Dash M, Padhi S, Mohanty I, Panda P, Parida B. Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J Family Community Med. 2013; 20(1):20–26.
[18] Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: a multicenter study. J Infect Dev Ctries. 2008; 2(5):354–358.
[19] Sood S, Gupta R. Antibiotic resistance pattern of community acquired uropathogens at a tertiary care hospital in Jaipur, Rajasthan. Indian J Community Med. 2012; 37(1):39–44.
[20] Nagaraj S, Kalal BS, Kamath N, Muralidharan S. Microbiological and antimicrobial profile of pathogens associated with pediatric urinary tract infection: A one year retrospective study from a tertiary care teaching hospital. Natl J Lab Med. 2014; 3(1):4–7.
[21] Eshwarappa M, Dosegowda R, Aprameya IV, Khan MW, Kumar PS, et al. Clinico-microbiological profile of urinary tract infection in south India. Indian J Nephrol. 2011; 21(1):30–36.
[22] Sharma I, Paul D. Prevalence of community acquired urinary tract infections in Silchar Medical College, Assam, India and its antimicrobial susceptibility profile. Indian J Med Sci. 2012; 66:273–279.
[23] Kaur N, Sharma S, Malhotra S, Madan P, Hans C. Urinary tract infection: Aetiology and antimicrobial resistance pattern in infants from a tertiary care hospital in northern India. J Clin Diagn Res. 2014; 8(10):1–3.
[24] Deshkar DW, Narute JV, Somvanshi VD. Bacteriological profile and antibiogram of uropathogens - A retrospective analysis. Int J Curr Microbiol App Sci. 2019; 8(8):2464–2471.
[25] Xu Y, Gu B, Huang M, Liu H, Xu T, et al. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J Thorac Dis. 2015; 7(3):376–385.
[26] Rangari AA, Sharma S, Tyagi N, Singh P, Singh G, et al. Antibiotic susceptibility pattern of bacterial uropathogens isolated from patients at a tertiary care hospital in Western Uttar Pradesh of India. Int J Curr Microbiol App Sci. 2015; 4(10):646–657.
[27] Thattil SJ, Santhosh S. Prevalence of UTI in different age groups in a tertiary care hospital and their antibiogram. Int J Contemporary Med Res. 2018; 5(1):3–6.
[28] Vecchi ED, Sitia S, Romano CL, Ricci C, Mattina R, et al. Aetiology and antibiotic resistance patterns of urinary tract infections in the elderly: A 6-month study. J Med Microbiol. 2013; 62:859–863.
[29] Kalal BS, Nagaraj S. Urinary tract infections: A retrospective, descriptive study of causative organisms and antimicrobial pattern of samples received for culture, from a tertiary care setting. Germs 2016; 6(4):132–138.