Full Text
Among intracranial vascular malformations the cavernous malformation is one of the rarest, with a necropsy incidence of between 0.39% and 0.53% [1, 2]. Since the introduction of MRI, cavernous malformations are diagnosed more often [3, 4]. Between 10%–23% are located in the posterior fossa with most in the pons [5, 6]. If these lesions bleed they can cause severe functional disturbances or death. Correct management of these lesions is therefore vital. In this study we describe the clinical experience with eight surgically treated brain stem cavernomas.
Materials and methods
Between January 2013 and September 2014, eight patients with cavernous malformation of the brain stem were referred to our institution and were treated surgically. There were three men and five women, age ranging from 13 to 53 years (mean 34 years), and seven had experienced one or more haemorrhages (Table 1). Our criteria for a haemorrhagic episode were acute onset of symptoms, manifestation of new neurological deficits, and corresponding findings in magnetic resonance imaging (MRI) demonstrating haemorrhage into the cavernous malformation or the adjacent brain stem parenchyma. The existence of haemosiderin deposits alone was not sufficient to define a haemorrhagic episode. The patients’ preoperative and postoperative neurological state was classified according to the Modified Rankin scale to achieve a grading of functional disturbances of daily life activities. Operative removal of the cavernous malformation was performed under standard microsurgical conditions, with neurophysiological monitoring, and, more recently, using neuronavigation and neuroendoscopy. Follow up included a complete neurological examination of all eight patients. The mean follow up period was 10 months (range 6 months–15 months).
Table 1: Patients gender, age and complaints of cavernous malformations.
|
No
|
Sex
|
Age
|
Location
|
Clinical
|
Hemorrhage
|
Surgery
|
Rankins
pre-op
|
Rankins post op
|
Follow up
|
|
1
|
F
|
19
|
Pons
|
CN
|
1
|
RMSO
|
3
|
2
|
12
|
|
2
|
F
|
20
|
Pons
|
CN, Cerebella
|
2
|
RMSO
|
2
|
1
|
8
|
|
3
|
M
|
21
|
Mid brain
|
CN
|
-
|
Far lateral
|
0
|
0
|
12
|
|
4
|
F
|
24
|
Pons-mid brain
|
LTS
|
1
|
RMSO, stereotactic
|
0
|
0
|
8
|
|
5
|
F
|
42
|
Mid brain
|
HCP, CN
|
1
|
RMSO
|
4
|
5
|
15
|
|
6
|
M
|
43
|
Pons
|
CN, LTS, cerebella
|
1
|
RMSO
|
0
|
0
|
6
|
|
7
|
F
|
48
|
Pons
|
CN, cerebella
|
1
|
MLS, sub occipital
|
1
|
2
|
8
|
|
8
|
M
|
53
|
Pons-mid brain
|
CN, LTS, cerebella
|
1
|
Combined
|
1
|
2
|
6
|
Abbreviations: CN: Cranial Nerve Palsy, LTS: Long Tract Sign, RMSO: Retro mastoid sub occipital craniectomy, MLS: Mid line sub occipital craniectomy.
Results
Clinical presentation
Seven patients had symptoms of one or more haemorrhages. One patient had two episodes of haemorrhage, six patients had one haemorrhage. Acute onset of symptoms and neurological signs were characteristic for a haemorrhage. The haemorrhage was followed in most patients by signs and symptoms such as vertigo, headache, and worsening of neurological deficits with progressive gait disturbances, cranial nerve paresis and long tract disturbances. Preoperative imaging included MRI-brain in all cases. Modified Rankins scale was used for assessment in follow up (minimum six months).
Surgical treatment and MRI findings
The operative approach was selected according to two general considerations: to minimise damage of the surrounding structures and to facilitate complete resection of the lesion. All operations were performed under standard microsurgical conditions with neurophysiological monitoring in assistance with neuronavigation and endoscopy.
For planning of the surgical approach MRI was evaluated for the exact location of the cavernoma, its relation to the bleeding cavity, and the proximity of the lesion to the pial surface of the brain stem. Pons (five patients) was most common location followed by mid brain. During surgery the cavernous malformation or the bleeding cavity was directly visible at the surface of the brain stem in four patients. In seven patients only mild to moderate haemosiderin staining of the pial surface was detected after exposure of the brain stem. In one patient the operative field seemed normal.
Postoperative course and outcome
Four of the eight patients operated on, had a new neurological deficit in the early postoperative period. In 3 of these 4 patients the new deficits were transient. One patient had persistent unilateral sixth cranial nerve dysfunction. At the last follow up all patients were alive. Compared with the preoperative neurological conditions the postoperative state was improved in five patients. The preoperative average Rankin score was 1.75 points. At the time of the last follow up the average postoperative score had improved by 0.63 points. In one patient cerebral reservoir was inserted.
Illustrative cases
Case 1
A 53-year-old man presented with acute onset of headache associated with a left hemi paresis, gait disturbance, and lower cranial nerve disturbances with one episode of haemorrhage. Brain MRI showed a cavernous malformation located in the pons & midbrain (Figure 1). A combined sub occipital craniectomy and occipital craniotomy, the pons & midbrain were exposed showing a circumscribed red-bluish area. After evacuation of the haematoma, the cavernous malformation was identified and completely resected. Last follow up at six months showed mild left hemiparesis and left mild cerebellar signs whereas disturbances of the lower cranial nerves had resolved completely (Rankin score 2). The haematoma is removed and the cavernous malformation is identified. After resection of the lesion the cavity is collapsed. Hemosiderin stained gliotic tissue surrounding the lesion is left behind.
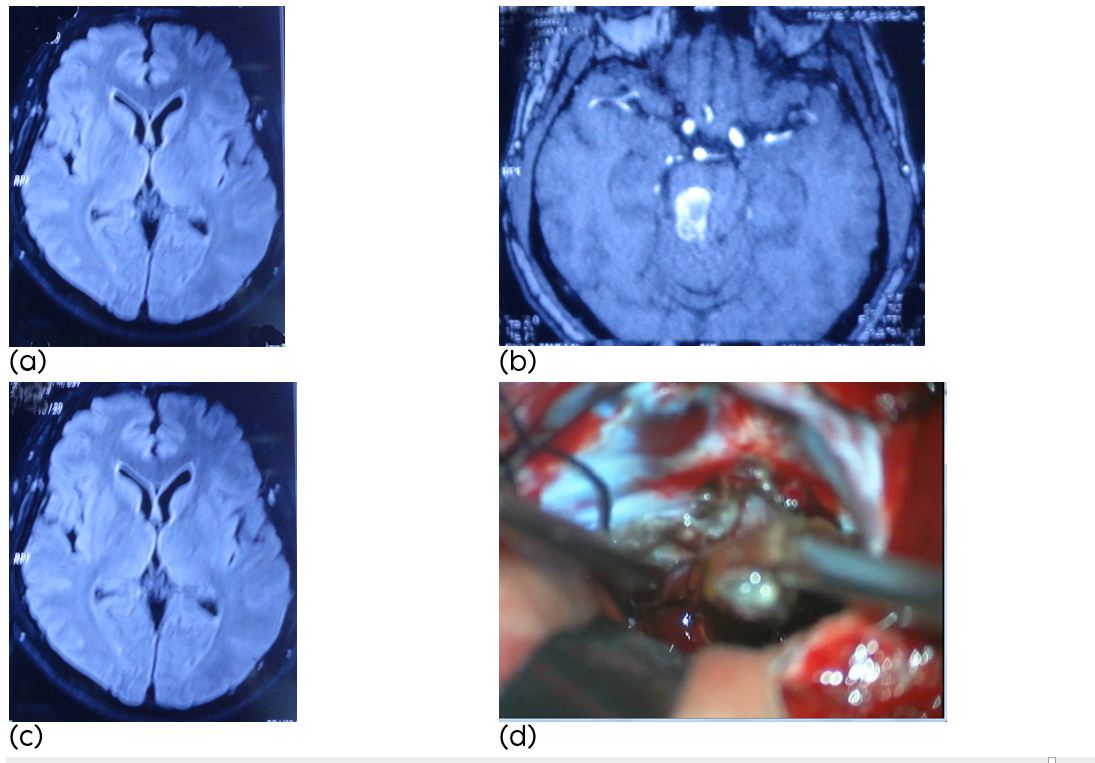
Figure 1a,b,c: (a-c) Axial MR image showing a cavernous malformation of the pons after the first haemorrhage, (d) Intraoperative photograph: After exposure the bulging aspect of the right lateral pons and subpial hemosiderin staining is visible.
Case 2
A 48-year-old man was admitted because of sudden onset of a left hemiparesis associated with headache, nausea and vomiting with involvement of lower cranial nerve involvement. Bells approach brain MRI demonstrated an acute intrinsic pontine haematoma (Figure 2). Retromastoidal cranieoctomy sub occipital trans cerebellar approach was chosen. The pial surface of the brain stem showed hemosiderin laden changes in colour. Using MRI guided navigation, the bleeding cavity and the cavernous malformation were localised and the entry point on the pial surface of the brain stem was identified. After complete excision of the cavernous malformation, the bleeding cavity was inspected with the neuroendoscope to exclude remaining pathological vessels and to achieve sufficient haemostasis. After eight months of surgery the neurological examination showed a residual left hemiparesis, but the patient was able to walk independently (Rankin score 2).
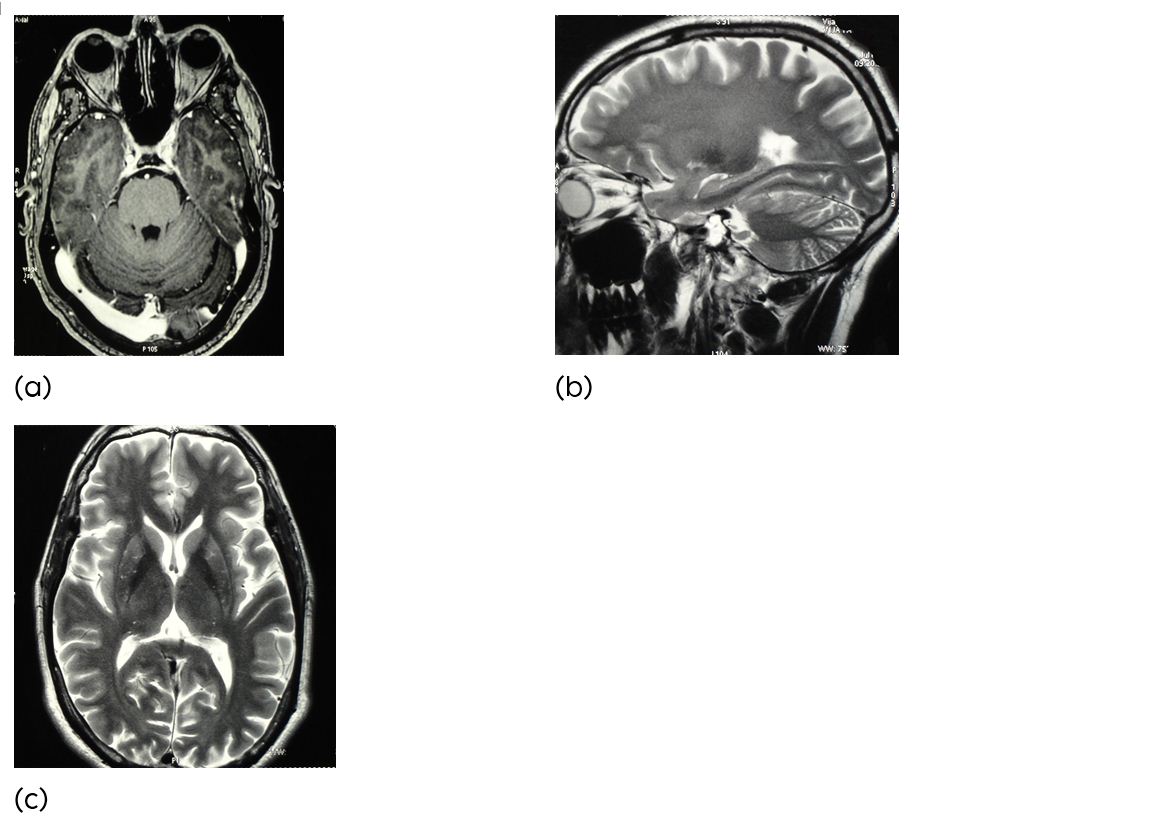
Figure 2a,b,c: (a-c) Axial & Sagital MR image showing an acute pontine haemorrhage.
Discussion
In recent decades the incidence of cerebral cavernous malformations (CCM) has increased due to diagnostic advances with widespread use of magnetic resonance imaging (MRI) in clinical practice (prevalence 0.4–0.9%) [7, 8]. Brainstem cavernomas account for 8–22% of all intracranial cavernomas. This subgroup of CCMs has a substantially higher propensity for bleeding (up to 30%), is more likely to result in severe neurological deficits [9-11] and moreover has a higher incidence of recurrent haemorrhage than those in other locations [12, 13]. Altogether, our experience with brainstem cavernoma includes over eight cases. Extent of persistent neurological deficits correlates with the number of recurrent haemorrhages, and rebleeding episodes tend to occur at progressively shorter time intervals. Haemorrhage from a brainstem cavernoma can be fatal in 20% of cases [9, 10, 12]. Neurological deficits depend realty on the localisation of the lesion and vary significantly, including various degrees of internuclear ophthalmoplegia, worsening hemiparesis, facial or abducens paresis, gaze palsy, facial, truncal and extremity numbness, dysphagia, dysarthria, and gait ataxia, among others [1, 10, 12]. The clinical symptoms usually appear in a subacute manner over hours or days, and most cases are treated temporarily with dexamethasone to avoid malignant swelling of the brainstem and secondary problems. Acute incidents with loss of consciousness or breathing disability occur very rarely.
Imaging
The gold standard for visualisation of the anatomical as well as pathological findings, such as the extent of the lesion and haemorrhage, is MRI. High field (1, 5 or 3, 0 Tesla) images with T1 (with and without contrast enhancement), T2 and gradient echo sequences in all three planes (axial, coronal, sagittal) are critical for guidance of all decisions. Additional tools such as T2-based imaging and fibre tracking have further improved the visualisation and understanding of this lesion [6].
Surgical treatment
Indications, goal and timing of surgery
Expert opinion varies regarding the indication and timing of surgery, but if haemorrhage appears associated with worsening of the neurological deficit surgical evacuation of the lesion and haematoma is recommended [14]. Exceptionally, surgery for even asymptomatic patients is also proposed. As a general rule clinical symptoms should be the main indication for surgery, and the patient option should preferably also be included in the decision process. The main goal of surgery is eliminating the risk of renewed haemorrhage and avoiding complications [1, 2, 12, 13]. Hence complete removal of the lesion is essential to prevent re-haemorrhage, which may occur in up to 43% of surgical cases [5]. However, in our brainstem cavernoma series we found a postoperative rebleeding rate of 4.4%. The risk of leaving residual portions of the lesion behind depends on surgeon experience. The larger the series, the lower the incidence of residuals [12, 13]. In the past two decades, waiting four to six weeks after a haemorrhagic event was recommended to stabilise the patients’ condition and waiting for the haematoma to become organised to achieve less active gliosis [15]. However, the incidence of rebleedings is highest within one month after surgery (21.8%) [12]. Prior to surgery, treatment with steroids for one or two weeks is recommended to resolve oedema and take advantage of haematoma cavity formation [13].
Surgical approaches to the brainstem and intraoperative monitoring
A great variety of surgical approaches, such as the suboccipital midline, retrosigmoid or subtemporal approaches exist in many instances of brainstem cavernoma [5, 12, 13, 15]. The choice of the proper approach depends on the relationship between the cavernoma and the pial or ependymal surface of the brainstem. As the floor of the fourth ventricle contains structures with important functions [3, 16] a lateral entry is preferred whenever possible.
Surgery of brain stem cavernomas has two main goals: to achieve complete resection of the lesion and to avoid additional neurological damage to the patient. As a first step after exposure of the lesion the surrounding haematoma is removed, and the cavernous malformation exposed and dissected. Care is taken not to penetrate the cavernoma but to work around the borders of the lesion, so that bleeding is minimised and dissection facilitated. After removal of the cavernous malformation, meticulous haemostasis is essential. No effort is made to remove the haemosiderin stained gliotic tissue that surrounds the cavity of the haematoma because it is unnecessary and may cause additional neurological damage.
Optimal timing of surgery is less well defined. In agreement with other authors [2, 15] we perform surgery in the subacute stage with a delay of several days or weeks after the haemorrhage, when the patient is in a stable condition. Additionally, in the subacute stage MR imaging allows better differentiation between the haematoma and the vascular malformation itself. Knowing the exact location of the cavernous malformation within the bleeding cavity is valuable for planning the surgical approach.
Neuronavigation
High resolution MRI is indispensable for the selection of the surgical trajectory to minimise or, if possible, completely avoid dissection through intact brain stem parenchyma. Patients with a deeply located cavernous malformation the use of neuronavigation is highly recommended to assist the surgeon in planning the incision. Some concern may arise about the reliability of navigation due to intraoperative brain shift. An important point is to use navigation in the very early stage of exposure. When applied with minimal brain retraction and before larger amounts of CSF were drained, precision of neuronavigation to localise the cavernous malformation is excellent in our experience.
Neuroendoscopy
Endoscopic assistance is valuable as the incision into the brain stem is kept as small as possible to avoid additional damage a keyhole is created in the depth of the operative field. The type of “deep keyhole” decreases the field of vision through the microscope significantly. Under these conditions a complete inspection of the resection cavity through the microscope is hardly achieved. With the endoscope near the keyhole using a 3 mm rigid wide angle lensscope a complete and detailed view of the entire cavity is obtained. Residual cavernous malformations or small bleeding points are easily identified with this technique.
Operative morbidity
The published outcome of surgically treated patients with brain stem cavernous malformation is generally good. In larger series surgical results were unchanged or improved in 69% to 91% [2, 10, 17]. Furthermore, there are several case reports of surgically treated brain stem cavernous malformations with excellent or good results [17, 18]. Whereas most authors had no surgical deaths, Porter et al. [12] reported a surgical mortality of 3.5%. Two of their patients died of cardiopulmonary arrest and one died of a haemorrhagic venous cerebellar infarction. Our results are comparable with the literature.
Indications for surgery
There is general agreement that patients with incidentally detected cavernous malformations are not surgically treated as long as the lesion produces no neurological symptoms by haemorrhage. However, if a first haemorrhage occurs surgical treatment in our opinion has to be considered, even if only mild neurological symptoms are present.
Some authors stated that patients with a previous haemorrhage are more likely to have a repeated haemorrhage [10, 12]. Porter reported a rehaemorrhage rate of 30%/ person/ year [12] and Fritschi et al. reported in their meta-analysis an average rebleeding rate of 21%/year/lesion [10]. In accordance with these studies we found a significantly higher risk of haemorrhage when the cavernous malformations already had caused symptoms by a previous haemorrhage. In our series the annual haemorrhage rate was 6.8% with a rate of 1.9 rehaemorrhage/ patient/ year. Furthermore, the literature and the findings in this series suggest that neurological deficits are more severe in repeated haemorrhages.
Complications, morbidity and mortality
Postoperative morbidity may be due to manipulation or oedema of brainstem parenchyma, and permanent morbidity was reported earlier in the range of 12% ~21% [12, 13, 19]. However, the morbidity rate is clearly related to surgical experience/ expertise [1, 2].
Conclusions
Brain stem cavernomas can be resected safely with surgical approach using meticulous microsurgical technique surgical approach. The additional use of modern tools such as neuronavigation, endoscopic assistance, and monitoring can contribute to the safety of the procedure. Goal of surgical intervention to achieve the complete resection of the lesion, without any neurological impairment.
Acknowledgements
Acknowledgements are due to the Departments of Radiology & Imageology, Krishna Institute of Medical Sciences (KIMS), Secunderabad.
Conflict of interest
Authors declare no conflict of interest.
References
1. Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U. Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002; 25(1-2):1–53.
2. Bertalanffy H, Gilsbach JM, Eggert HR, Seeger W. Microsurgery of deep-seated cavernous angiomas: report of 26 cases. Acta Neurochir (Wien). 1991; 108(3-4):91–99.
3. Bertalanffy H, Tissira N, Krayenbühl N, Bozinov O, Sarnthein J. Inter- and intra-patient variability of facial nerve response areas in the floor of the fourth ventricle. Neurosurgery, accepted for publication. 2011; 68(1 Suppl Operative):23–31.
4. Burkhardt JK, Schmidt D, Schoenauer R, Brokopp C, Agarova I, et al. Upregulation of transmembrane endothelial junction proteins in human cerebral cavernous malformation. Neurosurg Focus. 2010; 29(3):E3.
5. Cenzato M, Stefini R, Ambrosi C, Giovanelli M. Post-operative remnants of brainstem cavernomas: incidence, risk factors and management. Acta Neurochir (Wien). 2008; 150(9):879–886.
6. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009; 29(5):1433–1449.
7. Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991; 75(5):702–708.
8. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991; 75:709–714.
9. Ciurea AV, Nastase C, Tascu A, Brehar FM. Lethal recurrent haemorrhages of a brainstem cavernoma. Neurosurg Rev. 2007; 30(3):259–262.
10. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). 1994; 130(1-4):35–46.
11. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997; 87(2):190–197.
12. Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999; 90(1):50–58.
13. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol. 2003; 59(6):444–454.
14. Steinberg GK, Chang SD, Gewirtz RJ, Lopez JR. Microsurgical resection of brainstem, thalamic, and basal ganglia angiographically occult vascular malformations. Neurosurgery. 2000; 46(2):260–270.
15. Fahlbusch R, Strauss C, Huk W, Röckelein G, Kömpf D, et al. Surgical removal of pontomesencephalic cavernous hemangiomas. Neurosurgery. 1990; 26:449–457.
16. Recalde RJ, Figueiredo EG, de Oliveira E. Microsurgical anatomy of the safe entry zones on the anterolateral brainstem related to surgical approaches to cavernous malformations. Neurosurgery. 2008; 62(3 Suppl 1):9–15.
17. Kashiwagi S, van Loveren HR, Tew JM, et al. Diagnosis and treatment of vascular brain-stem malformations. J Neurosurg. 1990; 72(1):27–34.
18. Bellotti C, Medina M, Oliveri G, Barrale S, Ettorre F. Cystic cavernous angiomas of the posterior fossa: report of three cases. J Neurosurg. 1985; 63(5):797–7999.
19. Ferroli P, Sinisi M, Franzini A, Giombini S, Solero CL, et al. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery. 2005; 56(6):1203–12.