Full Text
Introduction
Heterotopic ossification (HO) is the abnormal process of calcification or ossification in muscles and other soft tissues. It is also called myositis ossificans, soft tissue calcification, dystrophic calcification and periarticular soft tissue calcification. The specific pathophysiology is not clear. It is the alteration in the ground substance of connective tissue progressing to ossification. Histologically, there is proliferation of fibroblasts and mesenchymal cells with inappropriate differentiation of fibroblasts into osteoblasts. Three forms are recognised: 1. A rare genetic disease called myositis ossificans progressiva, which name is changed to the modern term fibrodysplasia calcificans progressiva (FCP). 2. Neurogenic causes such as injuries of central nervous system, brain and spinal cord tumors and other neurodegenerative disorders of the brain. 3. Traumatic causes by accidents, surgery or burns. Heterotopic mineralisation is a better term as imageological differentiation between calcification and ossification is not easy.
Review of the literature and discussion
The pathology of myositis ossificans (MO) is essentially extra-osseous bone formation which occurs in muscle. It has a zonal organization with peripheral well organized mature lamellar bone, intermediate osteoid region and central immature non-ossified cellular focus. Radiology and other imaging methods play a major role in the early diagnosis of HO. Clinically, it may be mistaken for a neoplasm with a solitary or multiple painful swellings. Mobility of adjacent joint may be restricted.
Myositis ossificans progressive
Fibrodysplasia calcificans progressiva (FCP) is a rare congenital disorder characterized by malformations involving great toes and progressive heterotopic ossifications in muscles and soft tissues. Spontaneous mutation tends to be the cause in most of these cases. Genetic transmission is autosomal dominant with a receptor type of I/Activin-like kinase 2, and a bone morphogenetic protein (BMP) type I receptor. Atypical fibrodysplasia ossificans progressiva (FOP) patients also have heterozygous ACVR1 missense mutations in the amino acids [1-4].
Acquired myositis ossificans circumscripta
Acquired Myositis ossificans circumscripta is largely limited to a single muscle and normally one of mastication. This is generally caused by progressive ossification of intramuscular hematoma, which could occur due to a muscle injury. This term is used to classically describe non-hereditary forms of MO.
Most cases of HO occur as a result of trauma, and thus encountered in young adults. Another group which is particularly prone to MO include dystrophic and metastatic calcifications. In dystrophic calcification, the serum calcium is normal and calcium salts are deposited in damaged tissues, whereas in metastatic calcification, the serum calcium is high. The following table (Table 1) mentions the various causes of MO [5-8].
Table 1: Causes of ossification of the soft tissues and muscles.
|
Diseases of central nervous system
Diseases of peripheral nerves
Diseases of muscle
Miscellaneous – tetanus, hemophilia
Traumatic causes – accidents, post-surgical, burns, massage etc.
|
The causes of dystrophic calcification are mentioned in Table 2.
Table 2: Causes of dystrophic calcification.
|
Connective tissue disease
|
|
|
Progressive systemic sclerosis
|
|
|
Lupus and overlap connective tissue disorders
|
|
|
Dermatomyositis
|
|
Neoplastic diseases
|
|
|
Soft tissue sarcomas
|
|
|
Bone sarcomas
|
The metabolic causes of soft tissue calcification are mentioned in Table 3.
Table 3: Metabolic causes.
|
Hyperphosphatemia
Hypercalcemia
Hyperurecemia
|
Imaging characteristics
Myositis ossificans progressiva – Fibrodysplasia calcificans progressive (FCP)
Clinical suspicion of FCP in early life, on the basis of malformed great toes can lead to early clinical diagnosis with confirmatory diagnostic genetic testing. FCP can be evaluated using plain radiographs, CT and/or MRI. MRI is mostly useful for subtle oedema which would not be seen on the other modalities. Characteristic features include, hallux valgus, monophalangic first toe, shortened metacarpals, pseudoexostoses (ossification of ligamentous insertions), microdactyly of the first metacarpal/metatarsal, neck muscle oedema/calcification and C2-C7 facet joint fusion. Ossifications of various muscles of the trunk and abdomen are characteristic of FCP (Figure 1a, b).
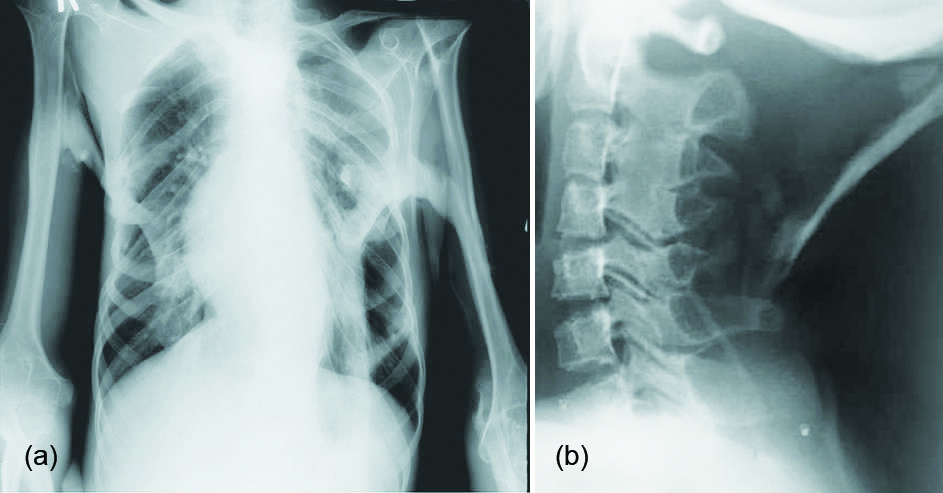
Figure 1: Fibrodysplasia calcificans progressive, myositis ossificans progressive: (a) Thorax, (b) Neck.
Heterotopic ossification (HO)
Conventional/ digital radiography is the study of first choice. Circumferential calcification – from periphery to centre with intermediate osteoid region. Similar presentation is noted in myositis ossificans circumscripta, FCP, panniculitis ossificans, and fibro-osseus pseudotumor of digits. The severity of heterotopic bone formation has been classified according to several systems. One traditionally used method is the Brooker classification system which divides severity into three grades a, b and c [7]. The important distinction in reporting the presence of HO is the presence of a space of more than 1 cm or less between opposing surfaces of bone and soft tissue.
Calcification usually begins to become apparent on plain radiographs within 2 - 6 weeks, and the lesion reaches the classic well circumscribed peripherally calcified appearance by 2 months. In the following 4 or so months, it typically becomes smaller and denser. Cleft between it and the subjacent bone may be difficult to see on plain films. CT appearances are similar to those of plain radiography, demonstrating mineralisation proceeding from the outer margins towards the centre. The cleft between it and the subjacent bone is usually visible. The peripheral rim of mineralisation is usually visible within 4 - 6 weeks.
MO in relation to bone surface is of three kinds. 1. Parosteal lesions may induce periosteal reaction without true bone attachment. 2. Lesions located closer to bone may show focal or broad-based attachment to bone surface. 3. The lesions attached to bone surface do not show continuous fusion with underlying cortex. Prominent cartilage metaplasia within lesion and in periosteal new bone formation can be present in the region corresponding to bone attachment.
The typical radiographic appearance of HO is circumferential calcification with a lucent centre, and a radiolucent cleft that separates the lesion from the cortex of the adjacent bone (Figure 2a-e). Localised post traumatic heterotopic mineralisation (Myositis ossificans):
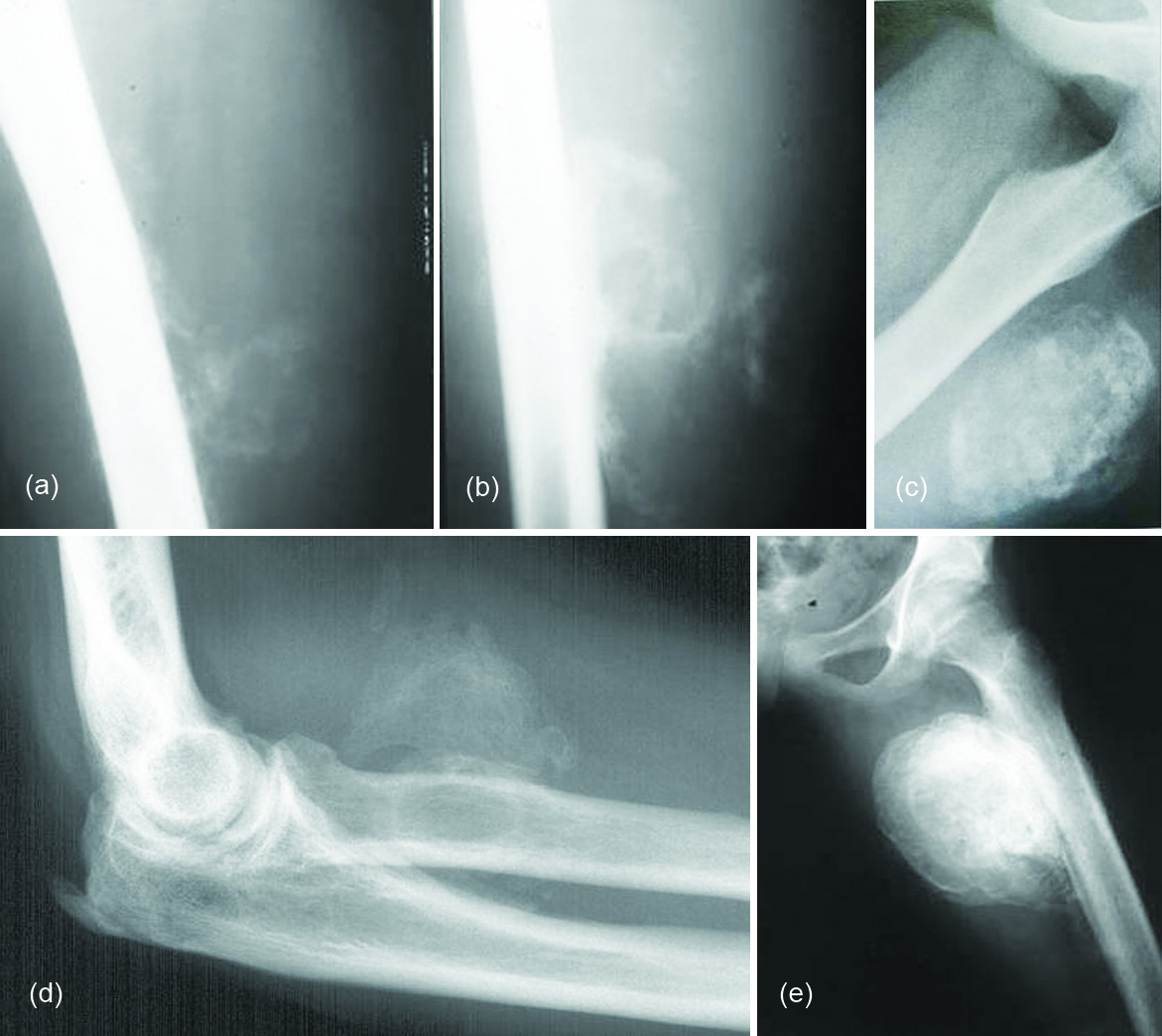
Figure 2: (a, b) Myositis ossificans in various stages in the thigh; (c) Post traumatic heterotopic ossification; (d, e): Post traumatic heterotopic ossification with periosteal reaction; (d) Elbow, (e) Thigh.
In early stages a typical finding is a soft tissue mass without calcific change. This can often be missed since radiographs are typically done for vague symptoms of pain. However, MRI may show edema, mineralisation can occur within 10 days after the causative insult. Calcification usually starts peripherally, though cases associated with FCP can calcify from the central zone out to the periphery lesions. It can be poorly organised without a recognisable mineralisation pattern. Mature cortical bone is formed if no treatment is used for the evolving heterotopic ossification [9-13].
Findings on CT mirror those of plain radiographs but CT is able to identify mineralisation earlier and has good overall specificity. It can sometimes be difficult to distinguish the soft tissue lesion seen early on in the evolution of HO from other causes of mineralisation. Serial imaging may be required to confirm the evolution of the lesion along the typical course for HO. In early stages low-attenuation soft tissue mass with indistinct surrounding soft tissue planes is observed in CT. It may show contrast enhancement. Zonal mineralisation pattern with a central fatty marrow component can occasionally be seen. Mature cortical bone occurs at the periphery. Post surgical soft tissue mineralisation occurs usually after five or six weeks (Figure 3a, b). In sports persons, avulsion injuries are quite common, which may result in tendinous, ligamentous and muscular calcifications which ossifies later. The ossifications around the hip may be segmental and simulate pelvic rib (Figure 4a, b). Post neurological HO generally occurs around the immobilized joints, such as hips and knees. This is also called parosteal arthropathy (Figure 5a-e). In India, massaging of the joints and muscles is common in post traumatic and inflammatory regions (Figure 6) which results in HO.
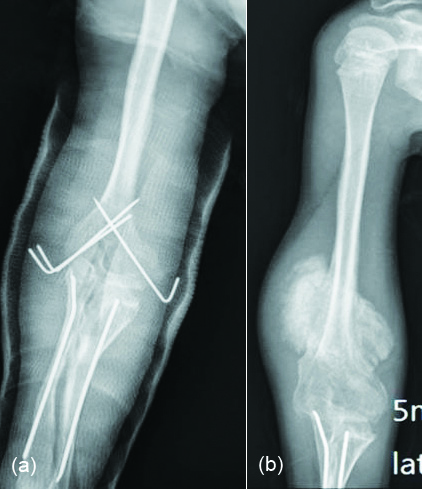
Figure 3a, b: Post operative heterotopic ossification.
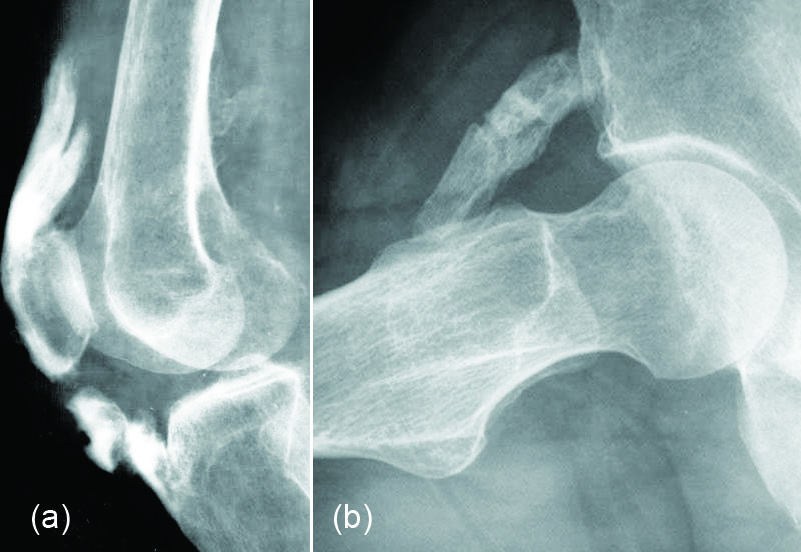
Figure 4: (a) Post avulsion injury with ossification of supra patellar and infra patellar tendons, (b) Pelvic rib.
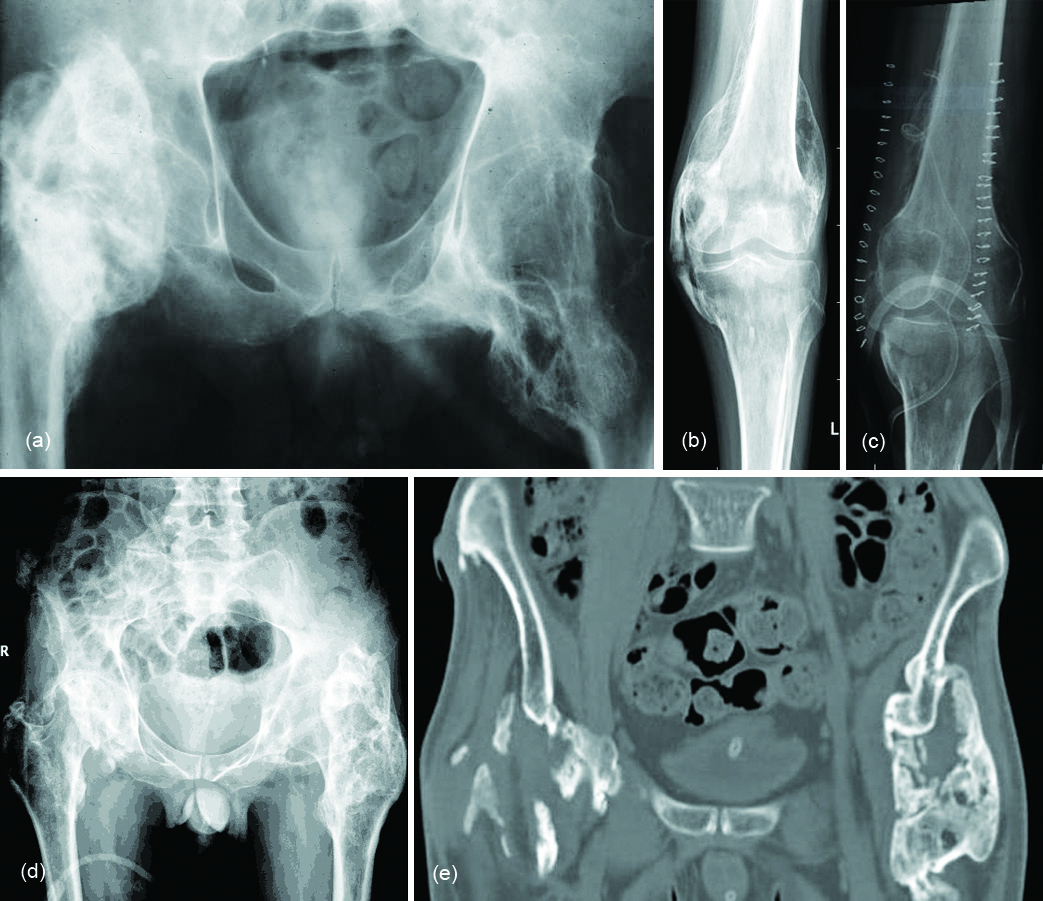
Figure 5: (a) Soft tissue ossifications in a patient with meningomyelocele; (b, c) Neurogical cause of heterotopic ossification: (b) Pre-op, (c) Post-op; (d, e): Paraosteo arthropathy in a paraplegia patient: (d) Plain film, (e) CT.
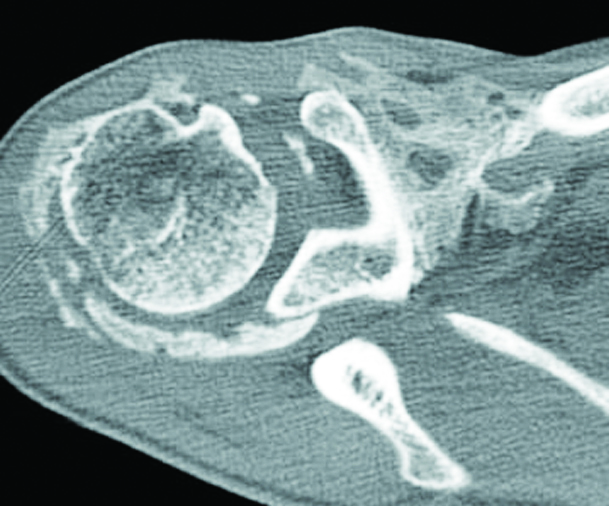
Figure 6: Post traumatic massage of the shoulder.
There is no specific role for MRI once the diagnosis of HO has already been made [14]. Instead, MRI is usually used in the assessment of a soft tissue mass. It has the added advantage of evaluating for other possible causes such as neoplasms (i.e. sarcoma) or underlying osteomyelitis. In early stages, a soft tissue mass with heterogenous high T2 signal is seen. The lesion may manifest simply as enlargment of an involved muscle. The surrounding ill defined high T2 signal represents oedema.
Ultrasonography detects changes in the soft tissues and mineralisation earlier than conventional radiography [15]. Nuclear bone scan is most sensitive for early detection. Biopsy of the lesion could lead to a false-positive diagnosis of osteosarcoma unless the clinical findings are taken into account and time is allowed for the lesion to mature.
In the differential diagnosis of HO, the following disorders may be considered [16] in Table 4.
Table 4: Differential diagnosis of HO.
|
1. Renal osteodystrophy
2. Tumoral calcinosis
3. Connective tissue disorders
- Scleroderma
- Dermatomyositis
- Lupus erythematosis
4. Calcinosis Universalis
5. Myonecrosis
6. Extra skeletal osteosarcoma
7. Parosteal osteosarcoma
8. Chondrosarcoma
9. Chronic venous insufficiency
10. Gouty arthritis
11. Burns [17].
|
Renal osteodystrophy
Renal osteodystrophy is a radiological term consisting of 1. Osteoporosis, 2. Osteomalacia, 3. Osteosclerosis, 4. Secondary hyperparathyroidism, 5. Soft tissue calcifications.
Radiologically lump like and homogenous calcifications are noted in the periarticular soft tissues (Figure 7 a, b).
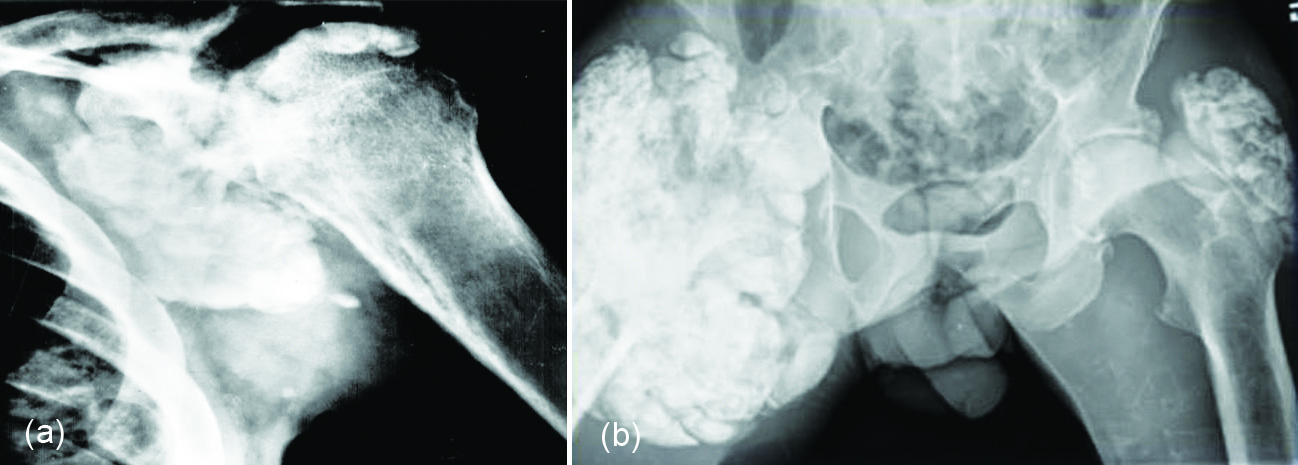
Figure 7: Renal osteodystrophy with tumoral calcinosis: (a) Shoulder, (b) Hips.
Tumoral calcinosis
Generally idiopathic, paraarticular with fluid – fluid levels in the dependent posture. Tumoral calcinosis is a rare entity characterized by localised extracapsular accumulations of calcium. Although, it is associated with renal osteodystrophy, idiopathic cases are also noted. The calcification begins as small, discrete nodules. However, they gradually enlarge and increase in density. The size varies between 1 – 20cms (Figure 8). Biopsy of the lesion shows hydroxyapatite calcium deposits with surrounding macrophages. Calcium levels may be normal in non-renal types [18].
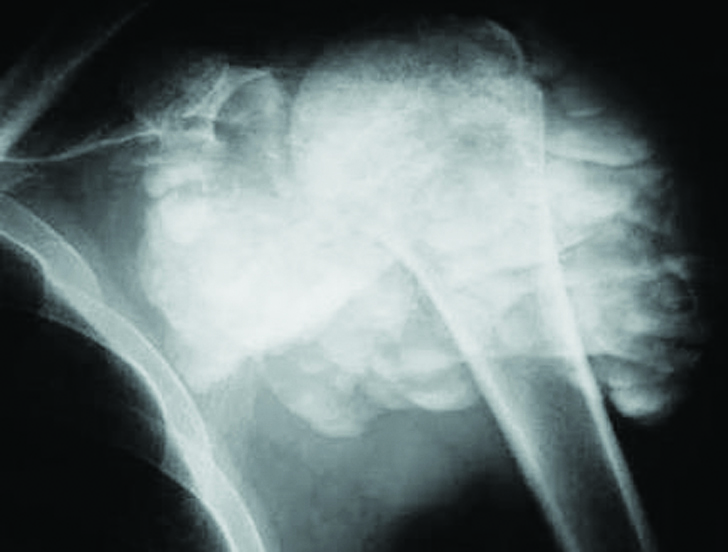
Figure 8: Tumoral calcinosis with fluid – fluid levels.
Connective tissue disorders
Scleroderma: In patients with scleroderma soft tissue calcification is often noted. It is estimated that about 75% of these patients show calcifications in the soft tissues of the hands. The pattern varies from punctuate to sheet like (Figure 9a). There may be subcutaneous and soft tissue calcifications elsewhere in the body (Figure 9b).
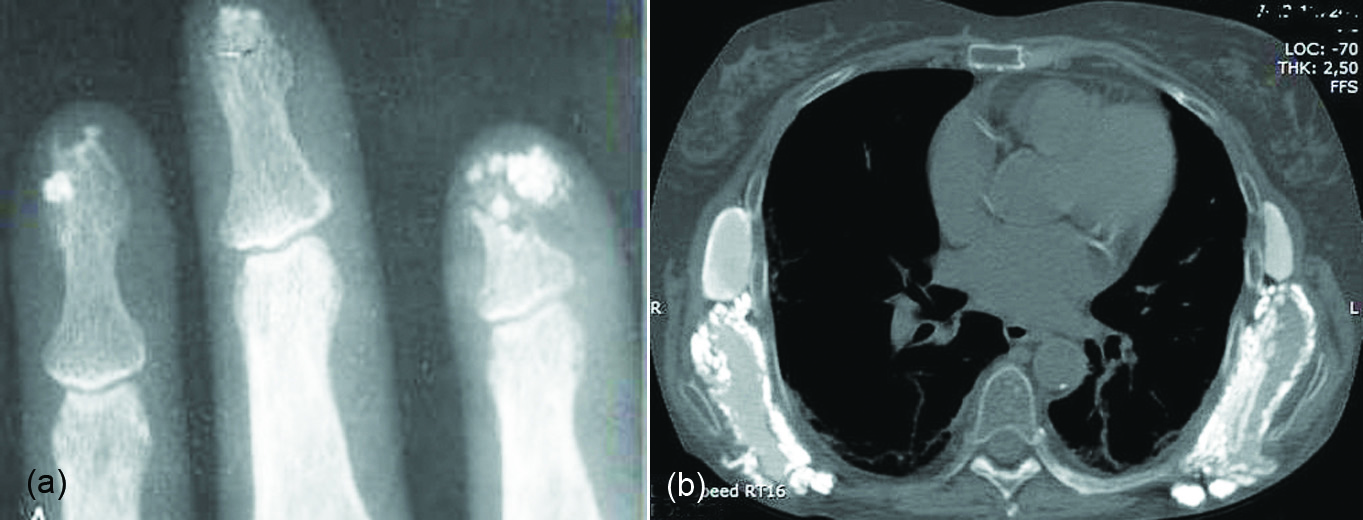
Figure 9: Scleoderma: (a) Calcifications at the tips of distal phalanges, (b) Thoracic wall.
Dermatomyositis: Clinically five types are noted (Table 5). In juvenile dermatomyositis, four different patterns of calcinosis have been described (Figure 10a-c).
Table 5: Clinical types of dermatomyositis.
|
1. Superficial plaques or nodules confined to the skin or subcutaneous tissue (33%)
|
|
2. Deep nodular deposits that extend to the muscles (20%)
|
|
3. Deposits along fascial planes of the muscles and tendons (16%)
|
|
4. Extensive hard calcium deposit that covers the entire surface of the body (10%)
22% combination
|
|
5. Calcinosis universalis
|
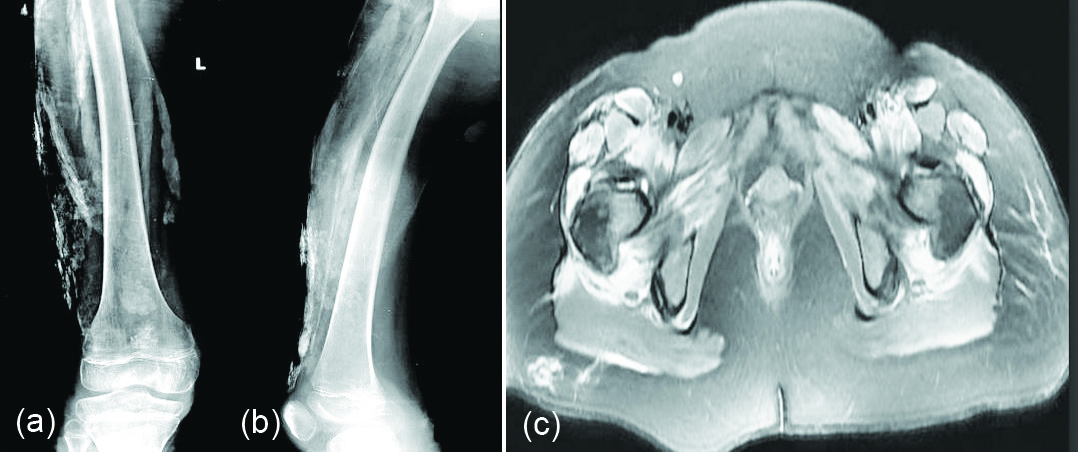
Figure 10: Dermatomyositis: (a, b) calcifications in the thigh, (c) MRI dermatomyositis calcifications in the soft tissues (white arrows).
Calcinosis universalis refers to diffuse, sheetlike deposition of calcium involving muscles, subcutaneous tissues and fascial planes. It may be idiopathic or associated with dermatomyositis and polymyositis (Figure 11).
Systemic lupus erythematosus: The calcification is not universal distribution in muscles and soft tissues.
Calcific myonecrosis: This is generally encountered in the muscles of compartments of the upper and lower limbs due to ischemia. Large sheets of calcification are noted following extensive myonecrosis [19] ( Figure 12).
Extraskeletal osteosarcoma
It is a rare malignant soft tissue tumor that produces osteoid. Two types exits: One is primary and the other is secondary to radiation exposure. It is rare in children. Radiographs reveal variable mineralization and half of these patients may show no calcification [20]. This can be differentiated from myositis ossificans by its homogeneity, dense ossification and central sclerosis. CT shows mineralization as well as necrosis of the lesion (Figure 13). MRI is nonspecific.
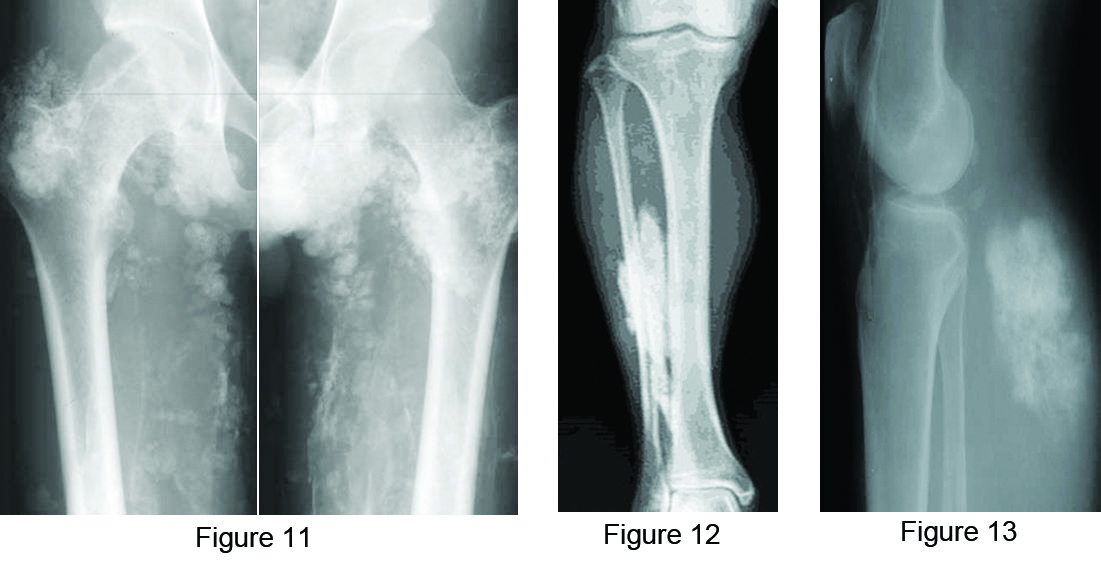
Figure 11: Calcinosis universalis – idiopathic.
Figure 12: Myonecrosis in the leg.
Figure 13: Extra skeletal osteosarcoma in the soft tissues of the calf.
Parosteal osteosarcoma
It is a localized bone producing tumor adjacent to the cortex. It occurs in 3rd to 4th decades of life. As opposed to myositis ossificans, the mineralization starts at the centre and spreads to the periphery. A radiolucent line is noted between the cortex and the ossifying mass. CT demonstrates the lucent line eminently (Figure 14a, b).

Figure 14: Parosteal osteosarcoma adjacent to the lower end of the femur: (a) plain film, (b) CT showing the lucent line.
Extra skeletal chondrosarcoma
Chondrosarcoma arising from soft tissues is very rare. Unless, there are typical ring-like or arc-like calcifications which indicate cartilaginous nature, it is difficult to make a radiological diagnosis. Even CT and MRI are not specific. Histology is only proof. The most common histological types of chondrosarcoma, myxoid and mesenchymal (Figure 15a-c).

Figure 15: Mesenchymal chondrosarcoma of the soft tissues of the thigh: (a) plain film, (b & c) MRI.
Chronic infections
Pyogenic, tubercular infections and parasitic infestations may show soft tissue calcifications. In chronic osteomyelitis, apart from irregular periosteal reaction soft tissue calcifications may also be seen (Figure 16a-c). In chronic tuberculosis of the skeleton, abcesses and granulomas may show calcification of the caseous material [21]. Parasitic calcifications is not included in this article.
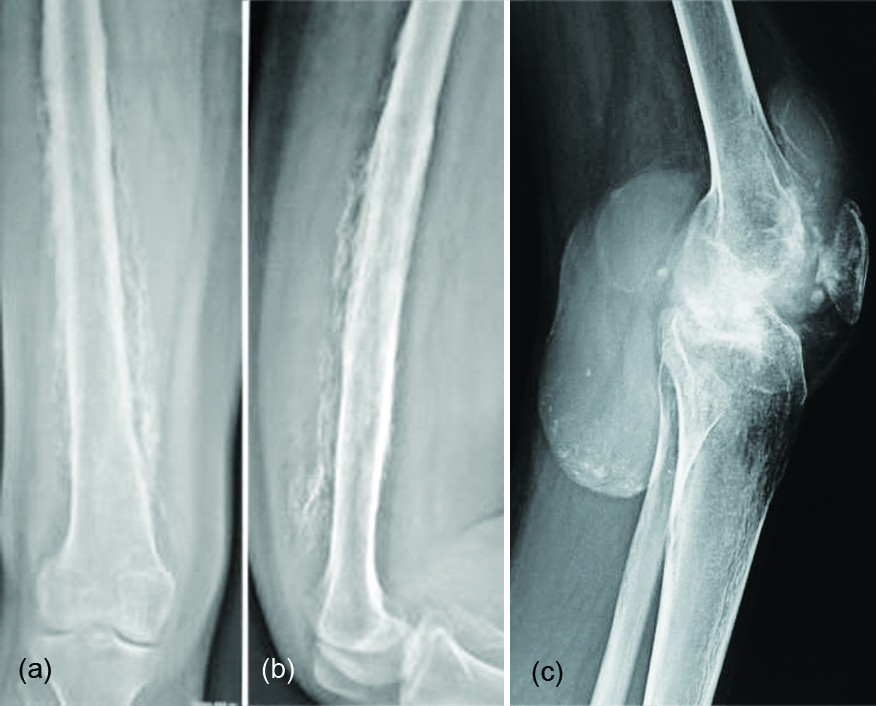
Figure 16: (a, b) Post infective heterotopic ossification in the soft tissues; (c) Heterotopic calcification in tuberculosis; popliteal abscess.
Chronic venous insufficiency
This often occurs in the veins of the legs due to incompetence of the valves. Subtutaneous dystrophic calcification with variable periosteal reaction is noted. The calcifications may be punctate, trabecular or reticular. Wavy type of periosteal reaction in the subjacent bones may also be noted (Figure 17).
Calcified hematoma: Chronic hematomas in the soft tissues either due to trauma or in patients with bleeding tendency may show variable types of calcification depending upon the age and stage. However, peripheral calcification is common. Ultrasonography CT and MRI confirm the findings (Figure 18a, b).

Figure 17: Chronic venous insufficiency with soft tissue calcification and periosteal reaction.
Figure 18: Calcified hematoma in the soft tissues of the back muscles: (a) MRI, (b) Ultrasonography.
Conclusion
Heterotopic ossification includes calcification and ossification. Imageologically, it is difficult to differentiate between calcification and ossification. Hence, the term mineralisation is preferred. Generally, calcification leads to ossification, but not vice versa. Several etiological factors are mentioned. The major causes include congenital and acquired. In the latter, trauma is the common cause. Neurogenic disorders also produce dystrophic calcifications. Metastatic calcification occurs when the serum calcium – phosphorus product is more than 75, calcium may precipitate in the soft tissues specially in the alkaline medium. The differential diagnosis includes extra skeletal osteosarcoma, parosteal osteosarcoma, renal osteodystrophy, tumoral calcinosis, collagen vascular disorders, chronic venous insufficiency, burns and chronic soft tissue hematomas.
Conflicts of interest
Author declares no conflicts of interest.
Acknowledgements
NIMS, KIMS, KREST Museum Hyderabad.
References
[1] Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011; 6:80.
[2] Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, et al. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008; 121(5):e1295–300.
[3] Shen Q, Little SC, Xu M, Haupt J, Ast C, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009; 119(11):3462–3472.
[4] Madhuri V, Santhanam M, Sugumar LK, Rajagopal K, Chilbule SK. Classical and atypical fibrodysplasia ossificans progressiva in India. Ann Hum Genet. 2015; 79(4):245–252.
[5] Zychowicz ME. Pathophysiology of heterotopic ossification. Orthop Nurs. 2013; 32(3):173–177.
[6] Gautschi OP, Cadosch D, Bauer S, Filgueira L, Zellweger R. Heterotopic ossification - from the aetiology to the current management. Unfallchirurg. 2008; 111(7):523–534.
[7] Della valle AG, Ruzo PS, Pavone V, Tolo E, Mintz DN, et al. Heterotopic ossification after total hip arthroplasty: A critical analysis of the Brooker classification and proposal of a simplified rating system. J Arthroplasty. 2002; 17(7):870–875.
[8] Hug KT, Alton TB, Gee AO. Classification in brief: Brooker classification of Heterotopic Ossification after total hip arthroplasty. Clin Ortho Relat Res. 2015; 473(6):2154–2157.
[9] Hacking C, Weerakkody Y. Heterotopic ossification. Available at https://radiopaedia.org/articles/heterotopic-ossification
[10] Ackman JB, Rosenthal DI. Generalised periart myosi ossi as a complication of pharmaceutically induced paralysis. Skeletal Radiol. 1995; 24:395–497.
[11] Ehara S, Shiraishi H, Abe M, Mizutani H. Reactive heterotopic ossification. Its patterns on MRI. Clin Imaging. 1998; 22(4):292–296.
[12] Jacobs JE, Birnbaum BA, Siegelman ES. Heterotopic ossification of midline abdominal incisions: CT and MR imaging findings. AJR Am J Roentgenol. 1996; 166(3):579–584.
[13] Ledermann HP, Schweitzer ME, Morrison WB. Pelvic heterotopic ossification: MR imaging characteristics. Radiology. 2002; 222(1):189–195.
[14] Wick L, Berger M, Knecht H, Glücker T, Ledermann HP. Magnetic resonance signal alterations in the acute onset of heterotopic ossification in patients with spinal cord injury. Eur Radiol. 2005; 15(9):1867–1875.
[15] Snoecx M, De Muynck M, Van Laere M. Association between muscle trauma and heterotopic ossification in spinal cord injured patients: Reflections on their causal relationship and the diagnostic value of ultrasonography. Paraplegia. 1995; 33(8):464–468.
[16] Banks KP, Bui-Mansfield LT, Chew FS, Collinson F. A compartmental approach to the radiographic evaluation of soft-tissue calcifications. Semin Roentgenol. 2005; 40(4):391–407.
[17] Evans EB, Smith JR. Bone and joint changes following burns; a roentgenographic study; preliminary report. J Bone Joint Surg Am. 1959; 41-A(5):785–799.
[18] Oslen KM, Chew FS. Tumoral calcinosis: Pearls, polemics, and alternative possibilities. Radiographics. 2006; 26(3):871–885.
[19] O'Dwyer HM, Al-Nakshabandi NA, Al-Muzahmi K, Ryan A, O'Connell JX, et al. Calcific myonecrosis: Keys to recognition and management. AJR Am J Roentgenol. 2006; 187(1):W67–76.
[20] Okada K, Ito H, Miyakoshi N, Sageshima M, Nishida J, et al. A low-grade extraskeletal osteosarcoma. Skeletal Radiol. 2003; 32(3):165–169.
[21] Resnick D. Diagnosis of bone and joint disorders. 2002. 4th ed., WB Saunders, Philadelphia. 2003; 58(5):412.