Review
2017
March
Volume : 5
Issue : 1
Imaging of langerhans histiocytosis - Pictorial essay
Subbarao Kakarla
Pdf Page Numbers :- 33-39
Subbarao Kakarla1,*
1KIMS Foundation and Research Centre, Minister Road, Secunderabad - 500003, Telangana, India
*Corresponding author: Prof. Kakarla Subbarao, MS, D.Sc. (HON), FRCR, FACR, FICP, FSASMA, FCCP, FICR, FCGP, Chairman, KIMS Foundation and Research Centre, Minister Road, Secunderabad - 500003, Telangana, India. Email: subbaraokakarla25@gmail.com
Received 13 October 2016; Revised 10 November 2016; Accepted 26 November 2016; Published 10 December 2016
Citation: Kakarla S. Imaging of langerhans histiocytosis - Pictorial essay. J Med Sci Res. 2017; 5(1):33-39. DOI: http://dx.doi.org/10.17727/JMSR.2017/5-7
Copyright: © 2017 Kakarla S. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Langerhans histiocytosis is the recent term used for the complex syndrome consisting of three sub types 1. Eosinophilic granuloma, 2. Hand-Schuller Christian disease and 3. Letterer-siwe disease. The clinical manifestations vary with each entity. Although rare, radiological findings present a typical pattern. Advanced Imaging adds to the plain film diagnosis. The Imaging findings are illustrated in detail.
Keywords: Langerhans histiocytosis; Imaging of eosinophilic granuloma; Hand-Schuller Christian disease; Letterer-Siwe disease
Full Text
Langerhans histiocytosis (LCH) is a complex syndrome with three subtypes, 1. Eosinophilic granuloma, 2. Hand-schuller Christian disease and 3. Letterer-siwe disease. It is a rare multi system disease and the incidence in India is not known. This term is replaced by the older term, Histiocytosis X. The etiology is unknown although, various theories have been proposed. Recent genomic studies showed activating, somatic BRAF mutations in most of the human specimens. This supports the concept of LCH as a myeloid neoplasm. Clinically the presentation depends upon the involved organ and the subtypes. Some of these may regress spontaneously and some of them have a rapid progressive course. In eosinophilic granuloma, there may be one or two skeletal lytic lesions. Around 70% of them are solitary. Although common in children, may be encountered in older age group also. The prognosis is good. In Hand-Schuller-Christian disease (HSC) clinically hepatosplenomegaly is noted with diabetes insipidus in some of the patients. Bone lesions are many. It is common in children and adolescents. The prognosis differs and depends on the severity of the lesions. In Letterer-Siwe disease, disseminated lesions are noted in skin, bones and lungs. It runs a Fulminant course with poor prognosis. Infants in young children are affected.
Review of the literature
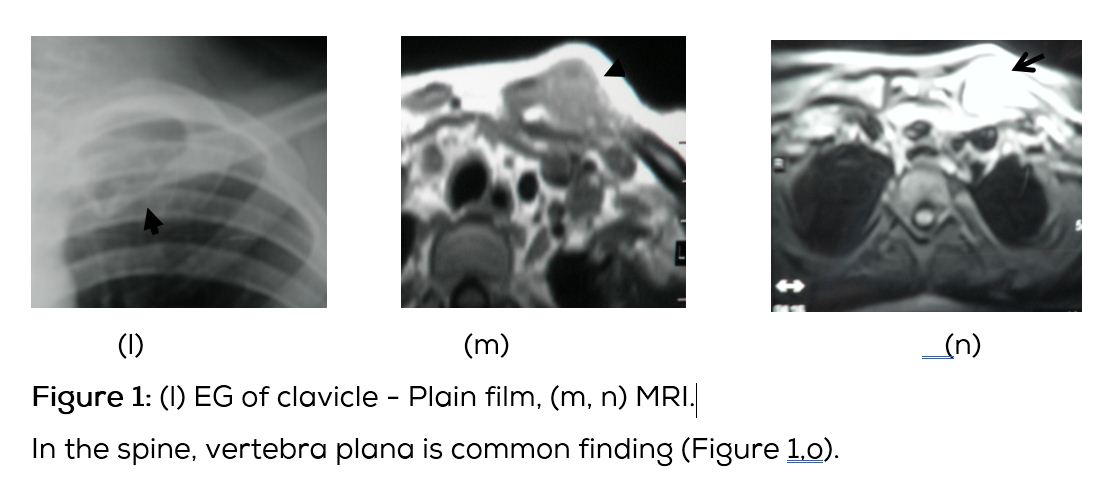
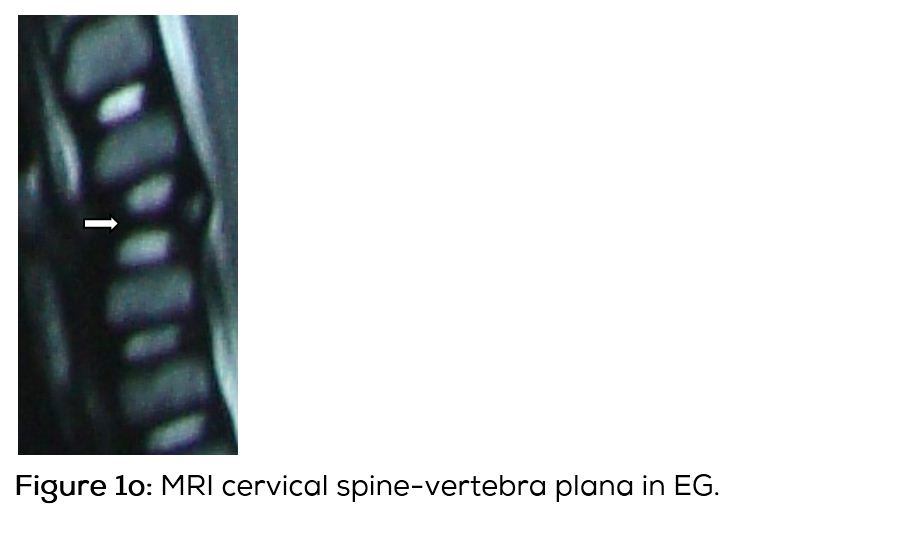
Langerhans histiocytosis (LCH) is a group of unknown disorders, characterized by the presence of cells with characteristics similar to bone marrow derived langerhans cells. In 1868, Paul lagerhans has discovered the epidermal dendritic cells, which bear his name [1]. The term LCH is preferred to the older term histiocytosis X, since its cellular basis has been clarified [2- 4]. The etiopathogenesis of LCH is unknown. However LCH has been recently classified as a myeloid neoplasm, since cancer associated gene mutations of the ERK pathway are found in this disorder [5, 6]. LCH predominantly affects children and young adults [7, 8]. When the bones are involved the skull is the most common site and has typical radiographic appearance. In the spine, vertebra plana is the classical appearance. In the long bones, a lytic lesion confined to medulla and encroaching the endosteum is noted. Benign type of periosteal reaction may also be present [9-11]. MRI plays a major role in differentiating eosinophilic granuloma with other disorders [12].
The radiological investigations include the following
· Conventional films
· MD CT
· MRI
· Scintigraphy
· Pet CT
Radiological findings in eosinophilic granuloma (EG)
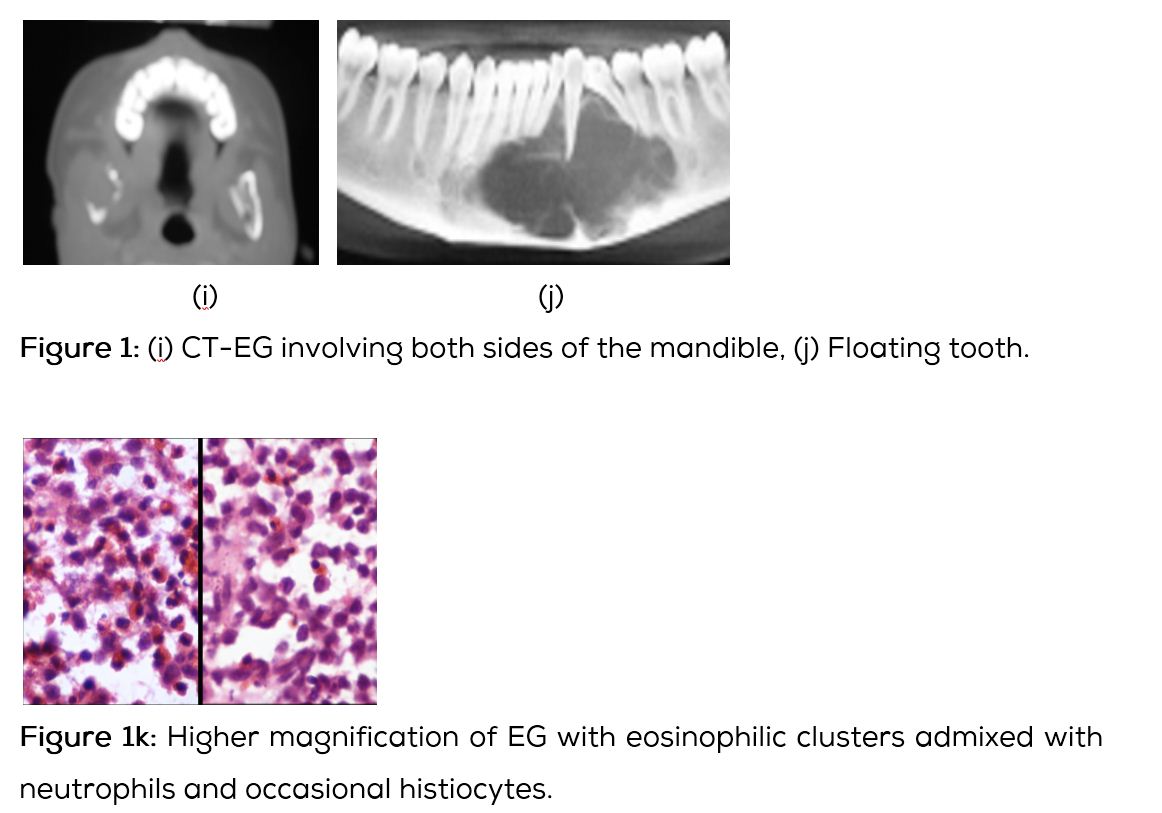
Conventional films show a lytic lesion of varying sizes between 1-3cms. However, multiple lytic lesions in the same bone or in multiple bones may be seen. In the long bones, lytic lesion is round or oval in shape with surrounding sclerosis and thick linear periosteal reaction (Figure 1a,b,c,d,e). In the flat bones such as skull, single or multiple large areas of lysis are seen. The bevelled edges in the skull are due to greater involvement of the inner than the outer table (Figure 1f,g). A button sequestrum is often noted in the lytic lesion (Figure 1h). The size of the lesion may be larger and multiple lesions may be seen. This appearance has been described as “geographical” areas of lysis. The differential diagnosis of multiple lytic lesions of bones of calvaria is listed in Table 1. In the mandible, areas of lysis are seen along the alveolar margin with “floating teeth” (Figure 1i,j). Histopathology shows clusters of eosinophilic cells with admixture of neutrophils and occasional histiocytes (Figure 1k). MRI is not specific but confirms the plain film findings (Figure 1l,m,n).
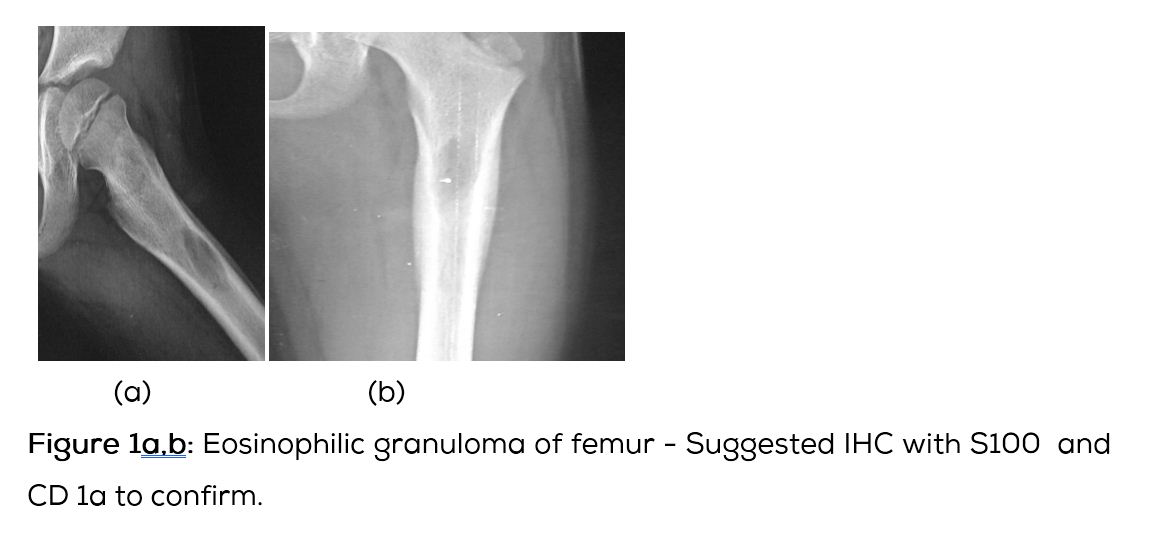
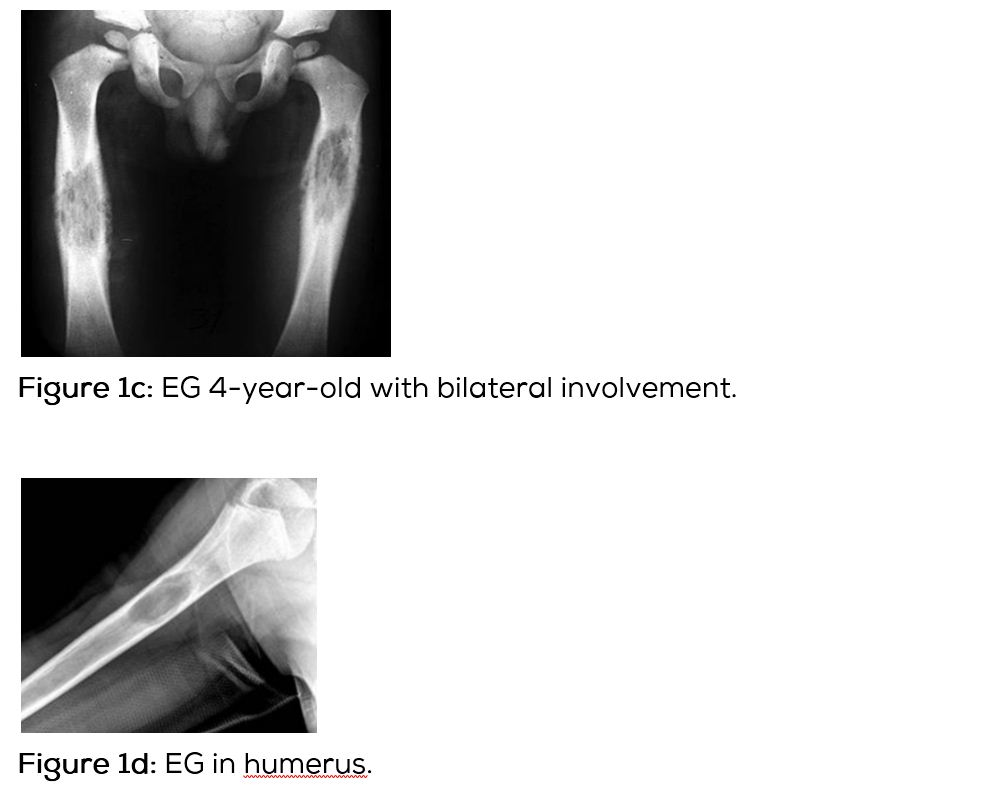
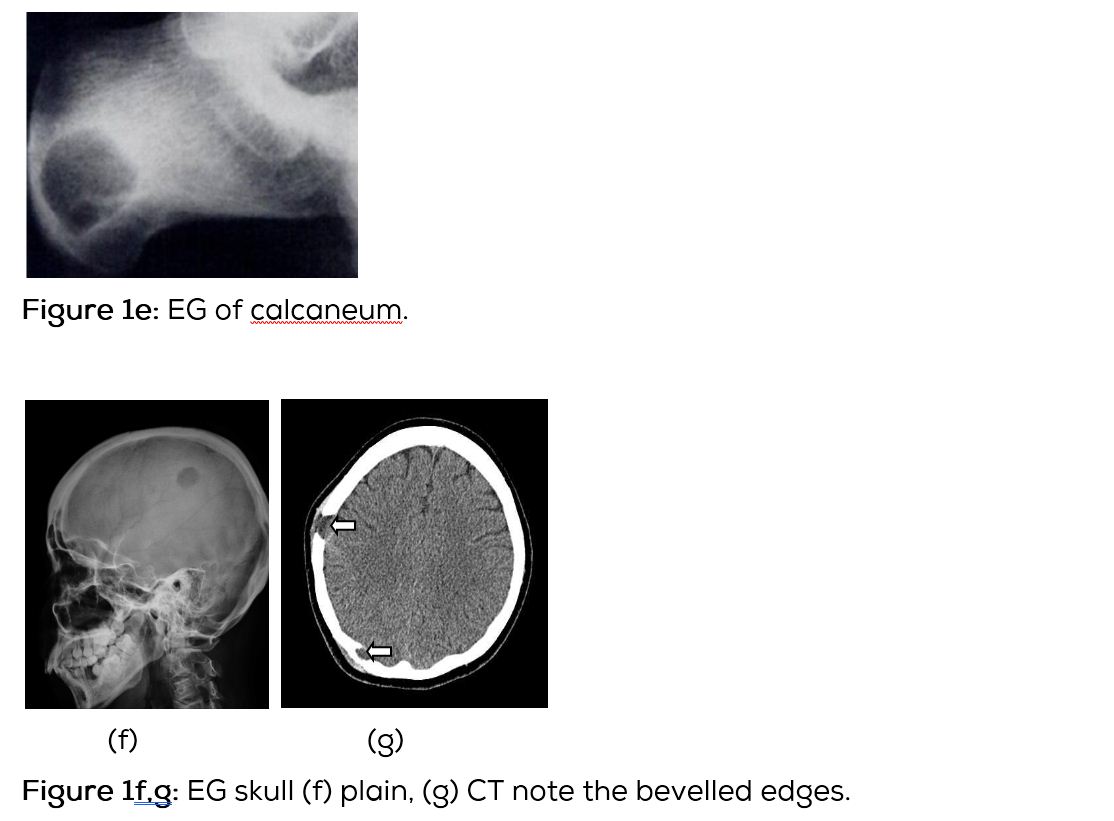
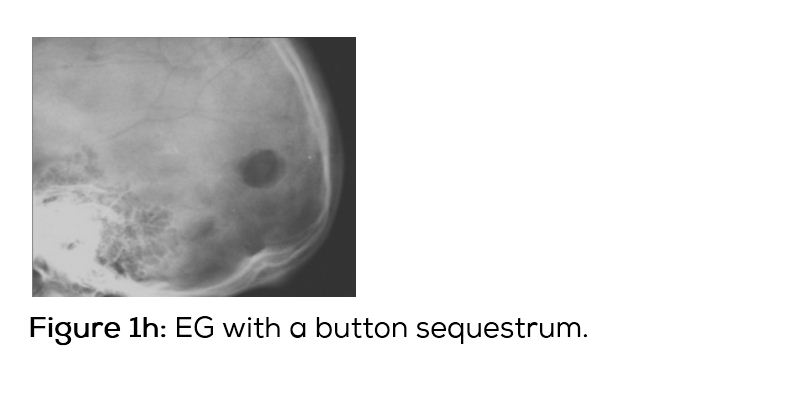
Table 1: Differential diagnosis of multiple lytic lesions of bones of calvaria.
|
• Langerhan’s histiocytosis
• Tuberculosis
• Osteomyelitis
• Hyperparathyrodism
• Lukemia/lymphoma
• Cystic angiomatosis
• Fibrous dysplasia
• Myeloma
• Metastasis
|
Radiology of Hand-Schuller Christian disease (HSC)
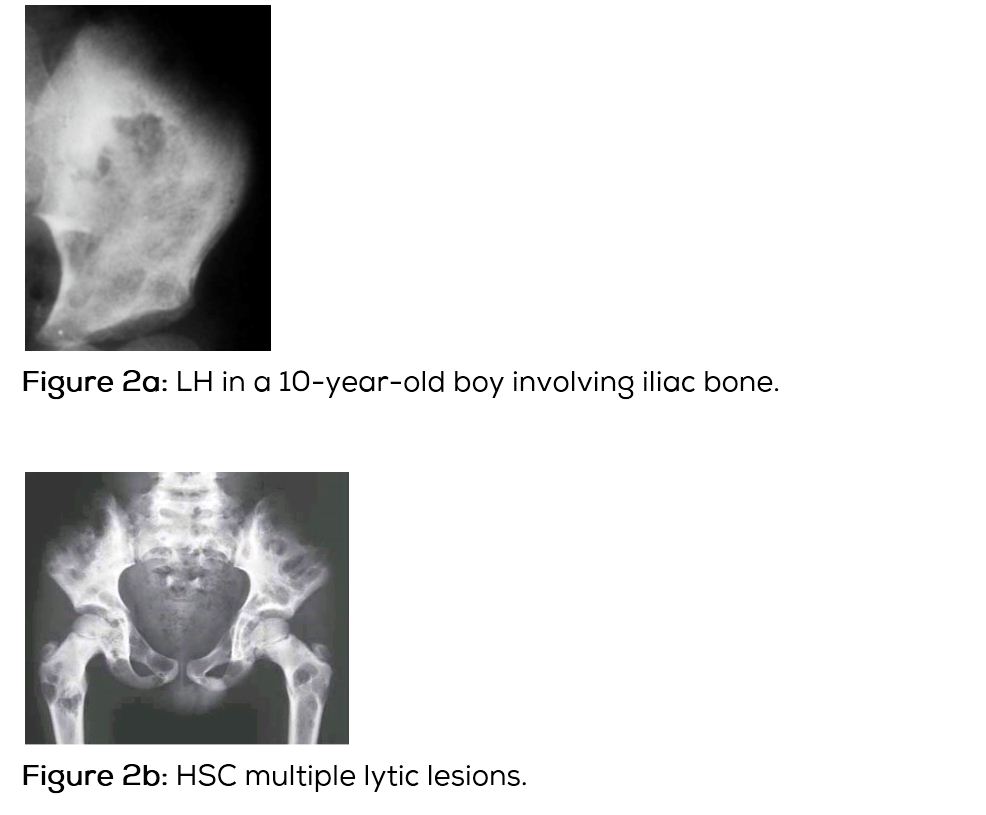
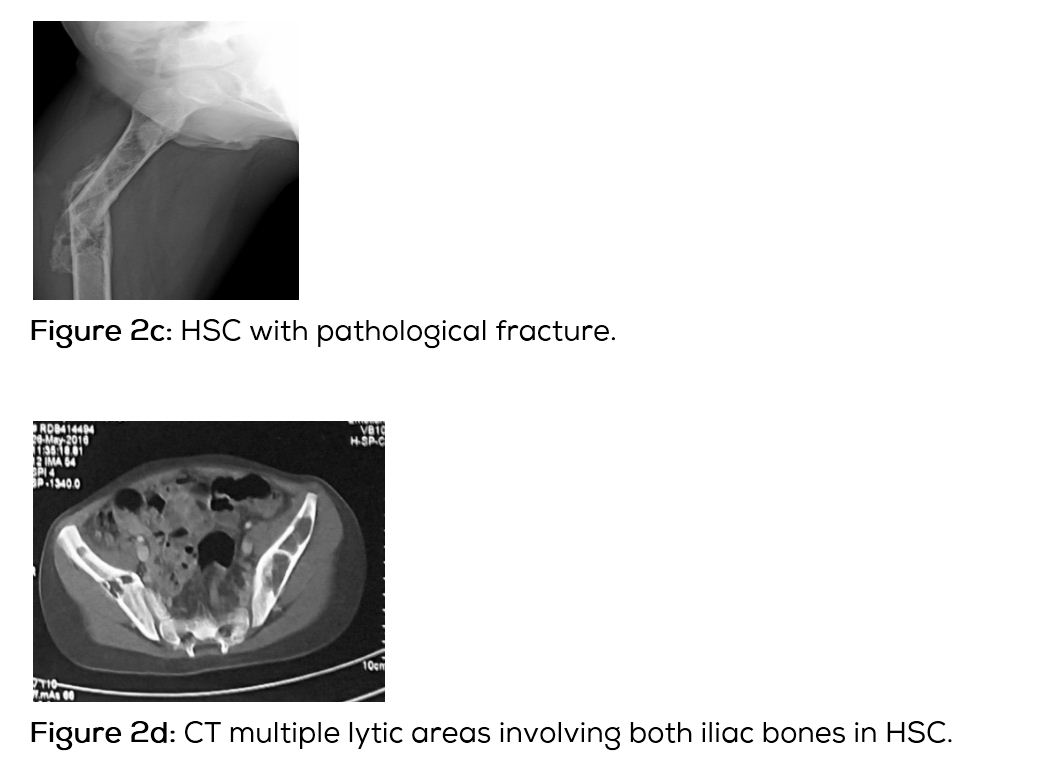
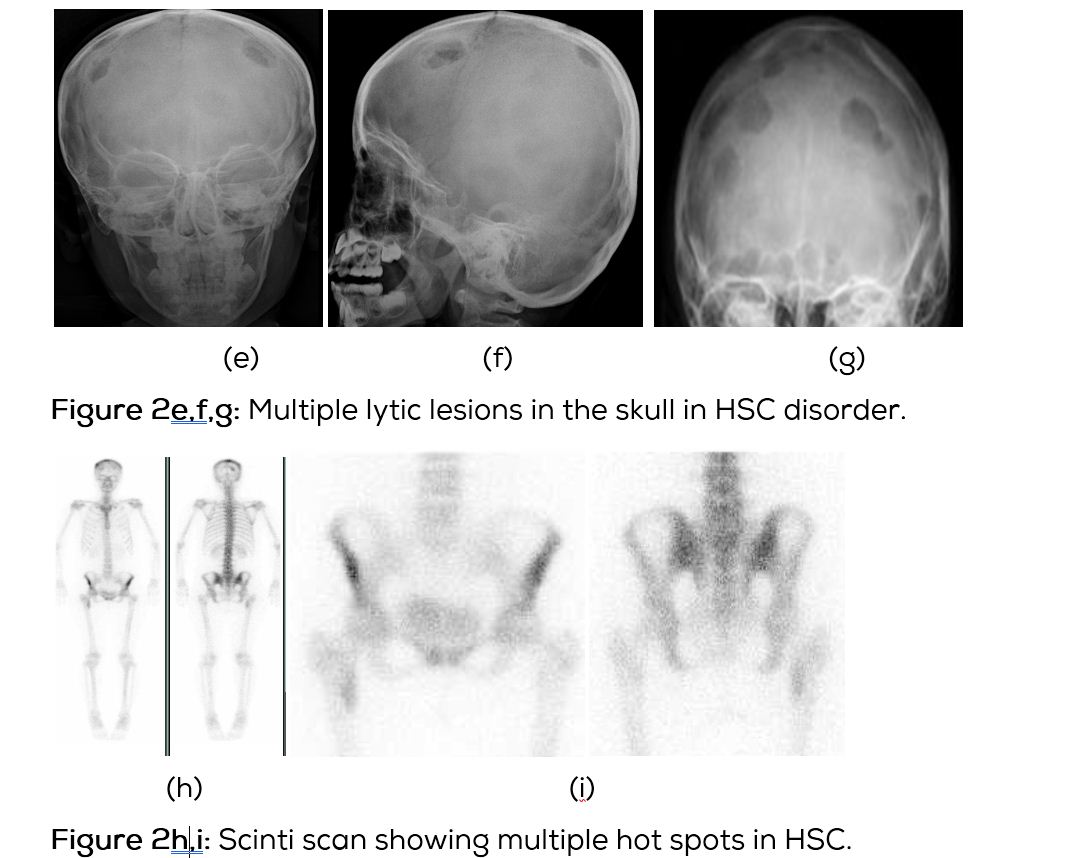
In this type of Langerhans histiocytosis, the skeletal lesions are multiple and disseminated associated with lymphadenopathy and hepatosplenomegaly. Interstitial pulmonary changes with mediastinal lymphadenopathy may be seen. Diabetes insipidus may occur. A chronic course may be seen. Radiological findings include multiple lytic lesions involving flat and long bones with areas of confluence (Figure 2a,b,c). CT shows the characteristic appearance with bevelled edges (Figure 2d). Skull shows lytic areas simulating eosinophilic granuloma (Figure 2e,f,g). Scintigraphy reveals multiple hot spots (Figure 2h,i).
Letterer-Siwe disease (LS)
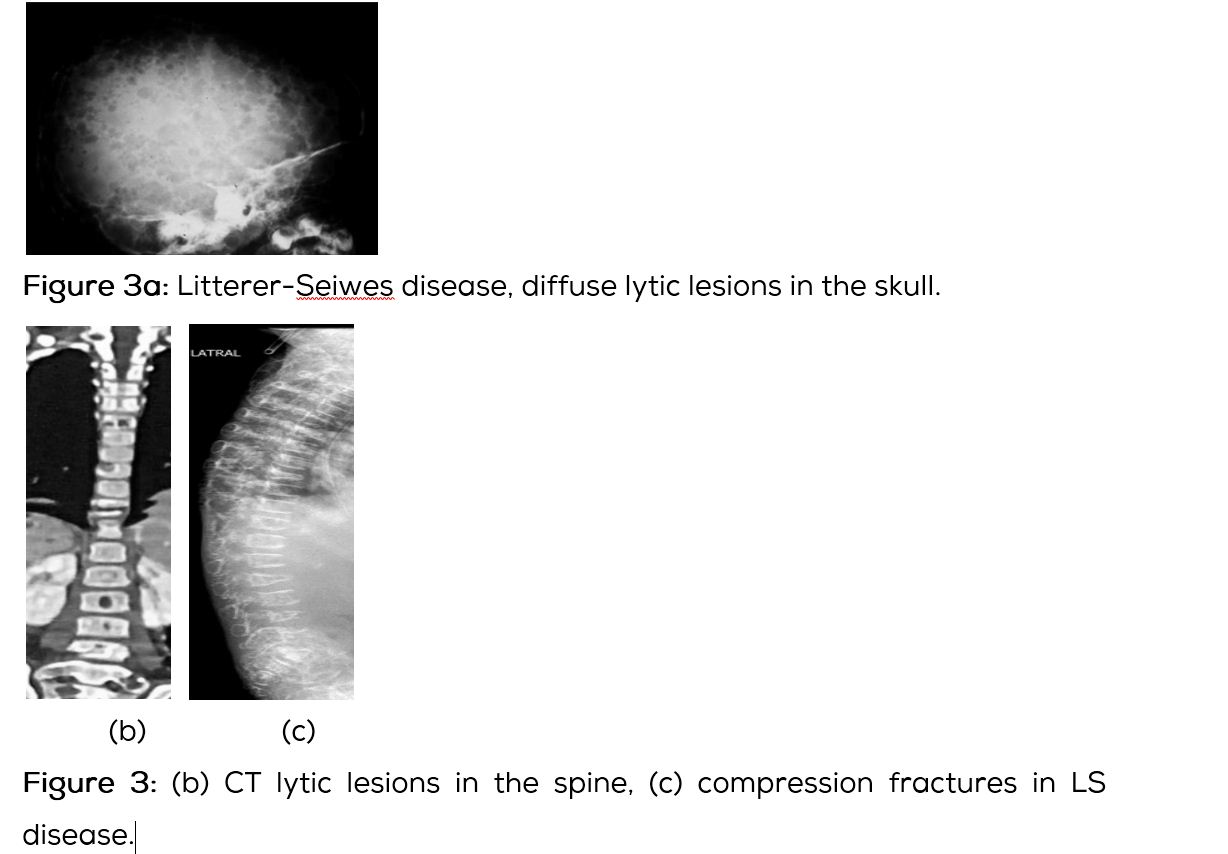
This subtype of LHS is often seen in younger age group below the age of 2 years. Clinically, the affected is quite ill presenting with skin rash, anemia leucocytosis, fever, mediastinal adenopathy and disseminated pulmonary infiltrates. The skeletal lesions are often wide spread simulating metastasis from neuroblastoma are leukemia (Figure 3a). With steroid and chemotherapies the children are surviving longer. Radiologically the skeletal lesions are similar to multiple diffuse lytic lesions in the skull and vertebra. Vertebra plana may be noted (Figure 3b,c). Chest radiograph shows diffuse interstitial infiltrates (Figure 3d).

Conclusion
Langerhans cell histiocytosis includes three subtypes 1. Eosinophilic granuloma, 2. Hand-schuller Christian disease and 3. Letterer-Siwe disease. The clinical findings differ with each type. Conventional radiography often reveals lytic lesions. Advanced imaging methods such as MDCT may help in charactersing the lytic lesion. MRI and scintigraphy help in revealing multiple lesions. Differential diagnosis of multiple lytic lesions of the skull is listed.
Acknowledgements
Departments of Radiology, NIMS, KIMS, and MNJ cancer and research centre.
Conflicts of interest
The author declares no conflict of interest.
References
[1] Komp DM. Historical perspectives of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1987; 1(1):9-21.
[2] Satter EK, High WA. Langerhans cell histiocytosis: A review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008; 25(3):291-295.
[3] Windebank KP, Nanduri V. Langerhans Cell Histiocytosis. Arch Dis Child. 2009; 94(11):904-908.
[4] Jaffe ES, Organization WH. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Iarc. 2001, ISBN:9283224116.
[5] El Demellawy D, Young JL, de Nanassy J, Chernetsova E, Nasr A. Langerhans cell histiocytosis: A comprehensive review. Pathology. 2015; 47(4):294-301.
[6] Badalian-Ver G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010; 116(11):1919-1923.
[7] Venkatramani R, Rosenberg S, Indramohan G, Jeng M, Jubran R. An exploratory epidemiological study of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2012; 59(7):1324-1326.
[8] Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans cell histiocytosis in adults. Med Pediatr Oncol. 1997; 28(1):9-14.
[9] Zaveri J, La Q, Yarmish G, Neuman J. More than just Langerhans cell histiocytosis: A radiologic review of histiocytic disorders. Radiographics: Rev Publ Radiol Soc N Am Inc. 2014; 34(7):2008-2024.
[10] Hashmi MA, Haque N, Chatterjee A, Guha S. Langerhans cell histiocytosis of long bones: MR imaging and complete follow-up study. J Cancer Res Therapeutics. 2012; 8(2):286-288.
[11] Murray Ronald O, Jacobson Harold G, Stoker Dennis J. The Radiology of skeletal disorders. Churchill Livingston, New York. 1995.
[12] Beltran J, Aparisi F, Bonmati LM, Rosenberg ZS, Present D, et al. Eosinophilc granuloma: MRI manifestations. Skelet Radiol. 1993; 23(3):157-161.