Short Communications
2013
March
Volume : 1
Issue : 1
Mechanical strategies to improve fluid over load in patients with cardiorenal syndrome
Murthy AS
Pdf Page Numbers :- 45-51
Murthy AS1,*
1Consultant Nephrologist, Krishna Institute of Medical Sciences, Minister Road, Secunderabad - 500003, AP, India
*Corresponding author: Dr. A. S. Murthy, MD, DM (Nephrology), Consultant Nephrologist, Krishna Institute of Medical Sciences, Minister Road, Secunderabad - 500003, AP, India, Mobile: +91 9000789665; E-mail: smthy85@yahoo.co.in
Received 10 December 2012; Revised 17 February 2013; Accepted 27 February 2013
Citation: Murthy AS. Mechanical strategies to improve fluid over load in patients with cardiorenal syndrome. J Med Sci Res 2013; 1(1): 45-51. http://dx.doi.org/10.17727/JMSR.2013/1-010
Copyright: © 2013 Murthy AS. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Treatment of acute deterioration in cardiac function traditionally involved diuretics, inotropes, and vasodilators. Usage of diuretics to reduce volume overload and pulmonary edema is often slow and many times accompanied by activation of RAAS and sympathetic nervous system with resultant increase in serum creatinine and heart rate. Other therapies that have been used in this setting include natriuretics and aquareticsalthough with limited success. To address this problem, mechanical removal of fluid (ultrafiltration/ aquapheresis) in volume overloaded patients is increasingly being done with lesser incidence of activation of RAAS and SNS. Both traditional dialysis machines and dedicated machines for isolated ultrafiltration have been employed.
Keywords: Ultrafiltration; Aquapheresis; Diuretics; Sympathetic nervous system (SNS); Renin angiotensin Aldosterone system (RAAS); Cardiorenal syndrome
Full Text
Introduction
Coexistent cardiac and renal involvement has been termed cardiorenal syndrome (CRS), either of which might have preceded or succeeded the other in acute or chronic form or both might have occurred simultaneously secondary to a common systemic process [1].
Patients with chronic kidney disease (CKD) are characterized not only by tendency to progress to end-stage renal disease (ESRD), but also by a high incidence of cardiovascular disease, more so in diabetics. Factors that are responsible for progression of kidney disease like hypertension, hyperglycemia, hyperlipedimia and proteinuria are also responsible for cardiovascular disease inthese patients. In addition, a host of other factors like hyperhomocystenemia, anemia, vascular calcification, uremic toxins and oxidative stress peculiar to CKD patients also contribute to the excessive cardiovascular mortality in these patients. Accelerated atherosclerosis and cardiomyopathy account for various manifestations of cardiovascular disease in these patients. In contrast to non CKD patients, coronary artery disease (CAD) in these patients manifests more frequently as sudden cardiac death and congestive heart failure [2].
Diuretics
Diuretics form the main stay of therapy for control of volume overload in patients with congestive heart failure(CHF) [3]. Of these, loop diuretics like Furosemide and Torsemide are the most frequently used agents, usually in combination with Potassium sparing diuretics like Spironolactone and Amiloride. Loop diuretics are associated with a classic doseresponse curve between the rate of diuretic excretion and the natriuresis [4]. Patients with CHF have a lesser response to a given dose than normal subjects for many reasons such as, decreased diuretic delivery to the kidney because of reduced renal blood flow, and increased sodium reabsorption at distal sites due to hypoperfusion-induced activation of the renin-angiotensin-aldosterone [4]. In addition, intestinal absorption of an oral loop diuretic may be delayed due to edematous state. These changes along with coexistent renal dysfunction require increasing doses of diuretics often as continuous infusions [5]. Thiazides may need to be added to loop diuretics to augment natriuresis.Loop diuretic effectiveness is often limited by coexistent hypoalbuminemia and proteinuria which increases diuretic resistance [6].
Potential complications of diuretics in patients with CHF
Though diuretics improve symptoms and signs related to fluid overload, this is often complicated by electrolyte imbalances (hypokalemia, hyponatremia) activation of RAAS, and sympathetic nervous system. These changes in the long term may translate in to adverse outcomes as observed in some studies [4, 7]. The evaluation study of congestive failure and pulmonary artery catheterization effectiveness (ESCAPE) trial [8], conducted to evaluate the use of pulmonary artery catheter in subjects admitted to the hospital with advanced heart failure, found a correlation between inpatient loop diuretic dose and adjusted 6-month mortality. In this trial 395 patients received diuretics in-hospital. A strong relation between dose and mortality was seen (p=0.003), especially at doses >300mg/day. (Figure 1).
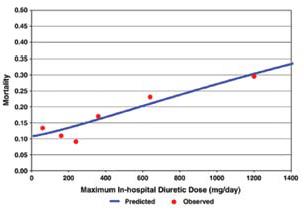
Figure 1: Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the ESCAPE trial [Hasselblad V, Gattisstough W, Shah MR, et al. (2007) Mortality as a function of maximum in-hospital diuretic dose].
Dose remained a significant predictor of mortality after adjusting for baseline variables that significantly predicted mortality. Using the acute decompensated heart failure national registry (ADHERE) [9], a large nationwide database of patients admitted to the hospital with heart failure, investigators were able to demonstrate that subjects receiving an inpatient intravenous dose of less than 160 mg of Furosemide equivalents had lower in-hospital mortality, fewer episodes of worsening renal function, and shorter length of stay than subjects treated with >160 mg of furosemide equivalents per day. Diuretic strategies in patients with acute decompensated heart failure (DOSE study) is a prospective, double-blind, randomized trial, where 308 patients with acute decompensated heart failure received furosemide administered intravenously by means of either a bolus every 12 hours or continuous infusion and at either a low dose (equivalent to the patient’s previous oral dose) or a high dose (2.5 times theprevious oral dose). There were no significant differences in patients’ global assessment of symptoms or in the change in renal function when diuretic therapy was administered by bolus as compared with continuous infusion or at a high dose as compared with a low dose [5, 10].
Mechanical methods of removing fluid
Apart from symptoms due to pulmonary congestion and low output state, congestive heart failure is often complicated by fluid accumulation in the abdomen leading to ascites. This is often worsened by coexistantnephrotic state due to underlying nephropathy and impaired glomerular filtration rate (GFR) adding to the respiratory compromise [1]. These are the patients who usually exhibit diuretic resistance [6].
Paracentesis
Removal of ascitic fluid by percutaeneous needle leads to marked improvement in symptoms due to reduction in intra-abdominal pressures and consequent improvement in renal function.This has been observedby Mullens et al. [11] in a small group of patients at the Cleveland clinic. In this study the renal and hemodynamic profiles of 9 consecutive, volume-overloaded subjects with acute decompensated heart failure (ADHF) andelevated intra-abdominal pressure (IAP), refractory to intensive medical therapy, were prospectively collected. All subjects experienced progressive elevation of serum creatinine and IAP in response to intravenous loop diuretics. Within 12 hours after mechanical fluid removal via paracentesis (n=5, mean volume removed 3187±1772 mL) or ultrafiltration (n=4, mean volume removed 1800±690 mL), there was a significant reduction in IAP (from 13±4 mm Hg to 7±2 mm Hg, P=.001), with corresponding improvement in renal function (serum creatinine from 3.4±1.4 mg/dL to 2.4±1.1 mg/dL, P=.01) without significantly altering any hemodynamic measurement. But given the chronic and recurrent nature of the underlying condition, repeated usage of paracentesis in these patients is not without the attendant risks of infection and possible fluid leaks.
Peritoneal dialysis
This modality has been conventionally used as a form of renal replacement therapy for patients with end stage renal disease ever since described byNolph et al. [12]. In this technique, a fluid (dialysate) of known composition is infused in to the abdomen through a soft catheter (Tenckoff) inserted in to the peritoneal cavity through a subcutaneous tunnel made in the anterior abdominal wall. Transport ofsolutes happens across the semi-permeable peritoneal membrane through the ultra-pores in themembrane [12] (Figure 2).
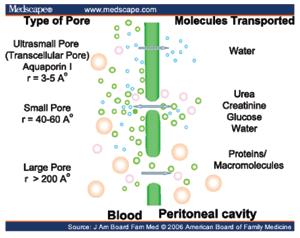
Figure 2: Peritoneal transports.
Ultrafiltration in thisprocesshappens due to the osmotic gradient exerted by hypertonic glucose in the peritoneal dialysis (PD) fluid. Given the compromised hemodynamic status of these patients with cardiorenal syndrome and the relatively noninvasive nature of the peritoneal dialysis, many patients with CRS have underwent peritoneal dialysis with good clinical outcomes as early as 1960s. Cairns KB 1968 , Mailloux LU [13]. In A single centre, prospective but non-randomized study in 20 patients with severe congestive heart failure, refractory to optimal pharmacological therapy [New York Heart Association (NYHA), class IV] was performed between 2000 and 2003 byGotloib et al. [14]. These patients had a baseline glomerular filtration rate of 14.84±3.8 ml/min. For all patients, the baseline ejection fraction was <35% (31.2±4.7%). Patients were treated initially by 2–5 sessions of continuous veno-venous haemofiltration (CVVH) or sequential haemofiltration (SHF). Automated peritoneal dialysis (APD) was started after implantation of a Tenckhoff catheter with three APD sessions/week (8 h each), with 15–20 l of dialysis fluid (PDF) per session. After 1 year of follow-up, all patients showed haemodynamic improvement: significant improvement of left cardiac work index, reduction of the systolic times, lower thoracic fluid contents, as well as a regression from NYHA class IV to class I. Need for hospitalization for CHF decreased from 157 to 13 days. Similarly Cnossenet al. [15] reported an acute rescue treatment of patients with treatment refractory heart failure. Functional improvement has been observed with the use of PD. However, fluid removal is less predictable compared with continuous haemofiltration therapies. Austin J Stack et al. [16] have compared the survival data in patients with ESRD treated by either of the modalities (HD, PD), from the National incidence data on 107,922 new ESRD patients from the Center for Medicare and Medicaid Services (CMS). These data suggest that peritoneal dialysis may not be the optimal choice for new ESRD patients with CHF perhaps through impaired volume regulation and worsening cardiomyopathy.
Despite these conflicting data PD still remains an important therapeutic option to expedite fluid and azotemic control in patients with cardiroenal syndrome, particulrly in the chronic setting than the acute decompensated state, when ultrafiltration/ aquapheresis is more appropriate.
Ultrafiltration/aquqpheresis/ hemofiltration/dialysis
Though these terms are often used to describe the extracorporeal removal of fluid in these patients, they actually indicate specific processes.
Ultrailtration or aquapheresis indicates removal of only fluid from the patient down the pressure gradient created by the machine (Figure 3).
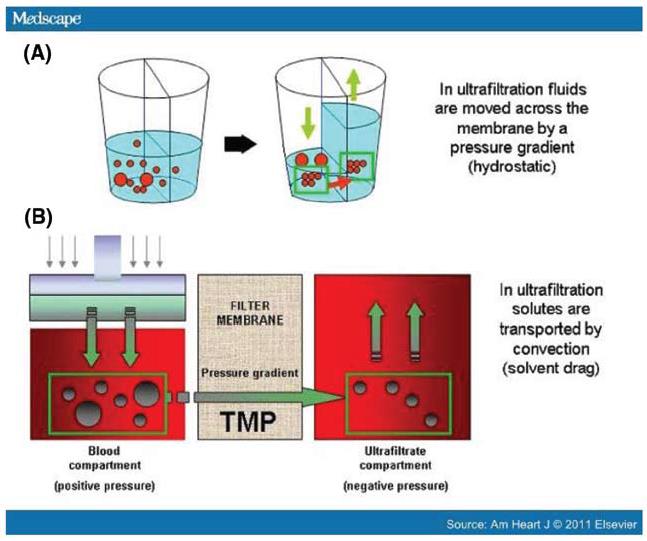
Figure 3A, B : Mechanism of ultrafiltration.
In hemofiltration the plasma ultrafiltrate is replaced by the substitution fluid of physiologic composition, ml per min. In the latter method, apart from fluid, solutes are removed by convection at isoosmolar concentration. In dialysis, apart from pressure driven UF, solutes pass to and fro in to the blood and the dialysate down their ‘concentration gradients driven by osmotic forces (Figure 4).
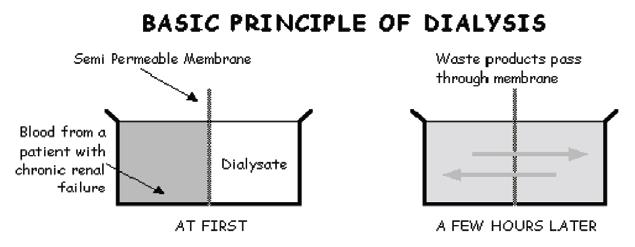
Figure 4: Basic principles of dialysis.
Fluid overload was treated in Patients with refractory CHF using the conventional dialysis machines and vascular access using the double lumen central venous dialysis catheters [17]. This has to be performed by trained dialysis technicians often in the setting of ICU. The extracorporeal volume of blood is usually around 200-250 ml and the blood flow is around 250-300 ml/min. These patients usually have difficulty in tolerating these regimens which are more suited for the conventional HD patient. Hence in these patients the blood flow rates are usually decreased, but flow rates below 100 ml/min are difficult to achieve in these machines. Costanzo et al. [18] has described role ofultrafiltration initiated within 4.7±3.5 h of hospitalizationand before IV diuretics were administered in 20 heart failure patients with volumeoverload and diuretic resistance. UF was achieved using peripheral venous cannulation. Re-evaluation was done at each hospital day, at 30 days, and at 90 days. A total of 8,654±4,205 ml were removed with ultrafiltration. Twelve patients (60%) were discharged in 3 days. One patientwas readmitted in 30 days. Weight (p=0.006), Minnesota livingwith heart failure scores (p=0.003), and Global Assessment (p=0.00003) improved after ultrafiltration and at 30 and 90 days. Median B-type natriuretic peptide levels decreased afterultrafiltration (from 1,230 pg/ml to 788 pg/ ml) and at 30 days (815 pg/ml) (p=0.035). Blood pressure, renal function, andmedications were unchanged. Now portable dedicated machines for prolonged ultrafiltration are available. They can used in non-dialysis areas and they function at low blood flow rates (40-50 ml/min) permitting use of peripheral venous access. They have been used in various clinical trials [19].
The UNLOAD [19] trial is the firstprospective, randomized and controlled study that enrolled 200 patients at as many as 20 U.S. clinical sites. In this trial, patients with heart failure were randomized to receive either intravenous diuretics or aquapheresis/ultrafiltration. Uf rate was upto 500 mls/min using peripheral venous access and a portable ultrafiltration system. After 48 hours, patients receiving aquapheresis had the following results (Table 1).
Table 1: Primary and secondary end points, ultrafiltration vs standard diuresis in UNLOAD trial.
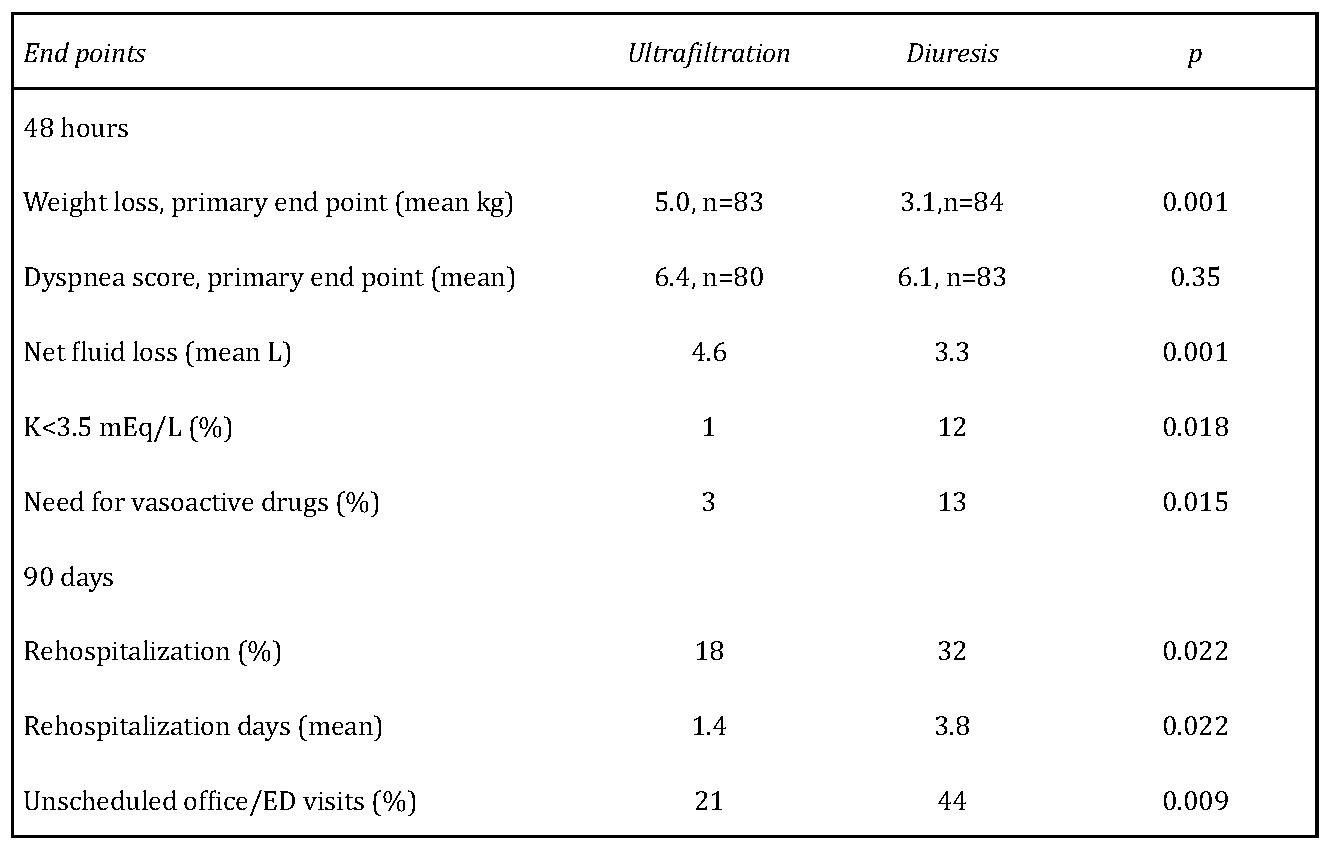
K=potassium; ED=emergency department:
- 38 % greater weight loss over standard care
- 28 % greater net fluid loss over standard care Equal improvement in dyspnea score ninety days after hospital discharge, patients receiving aquapheresis showed
- 43% reduction in patients requiring rehospitalizations for heart failure over standard care
- 50% reduction in the total number of rehospitalizations for heart failure over standard care
- 52% reduction in emergency department or clinic visits over standard care
- 63% total reduction in days re-hospitalized for heart failure over standard care
This study suggests that aqua filtration is an alternative therapy for hospitalized patients with heart failure that may be more effective than standard therapy.
Rate of fluid removal is always a matter of concern in these patients as they are prone to hypotension during the procedure due to myocardial dysfunction and autonomic neuropathy. Such episodes may further compromise the renal function. Hence the rate of ultrafiltration should match the rate of vascular refill from the interstitial compartment. While on most studies the UFR varied from 100 -500 ml/min, the magnitude of elevation of serum creatinine was greater (>0.5mg/dl) in those patients with higher UF rates (325±117mls/hr for 37.5±24.7 hrs) [20].
Conclusion
Ultrafiltration can be a safe and effective way of treating fluid overload in patients with CHF particularly in those with coexistent CKD and diuretic resistance. This is accompanied by fewer complications like activation of SNS, RAAS and worsening of renal failure. Both conventional methods of UF as in dialysis or the newer dedicated ultrafiltration devices using peripheral venous access can be employed to relieve congestive symptoms in these patients.
Conflicts of interest
Author declares no conflicts of interest.
References
1. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008, 52(19):1527-39.
2. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am SocNephrol 1998, 9:S16-23.
3. Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int 1984, 26(2):183-189.
4. Ikram H, Chan W, Espiner EA, Nicholls MG. Haemodynamic and hormone responses to acute and chronic frusemide therapy in congestive heart failure. Clin Sci 1980, 59(6):443-9.
5. Dormans TP, Van meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P. Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol 1996, 28(2):376-82.
6. Kirchner KA, Voelker JR, Brater DC. Intratubular albumin blunts the response to furosemide-A mechanism for diuretic resistance in the nephrotic syndrome. J Pharmacol Exp Ther 1990, 252(3):1097-101.
7. Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990 82(5):1724-9.
8. Hasselblad V, Gattisstough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007, 9(10):1064-1069.
9. Peacock WF, Costanzo MR, De marco T, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology 2009, 113 (1):12-9.
10. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011, 364 (9):797-805.
11. Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, Tang WH. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail 2008,14(6):508-514.
12. (KhannaNolph) Khanna R, Nolph KD, Principles of Peritoneal Dialysis Dialysis as Treatment of End-Stage Renal Disease, Chapter 4.
13. Mailloux LU, Swartz CD, Onesti G, Heider C, Ramirez O, Brest AN. Peritoneal Dialysis for Refractory Congestive Heart Failure. JAMA: The Journal of the American Medical Association 1967, 199(12):873-878.
14. Gotloib L, Fudin R, Yakubovich M, Vienken J. Peritoneal dialysis in refractory end-stage congestive heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant 2005, 20:vii32-6
15. Cnossen N, Kooman JP, Konings CJ, Van dantzig JM, Van der sande FM, Leunissen K. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant 2006, 21 Suppl 2:ii63-66.
16. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int. 2003, 64(3):1071-1079.
17. Simpson IA, Rae AP, Simpson K, et al. Ultrafiltration in the management of refractory congestive heart failure. Br Heart J. 1986, 55(4):344-347.
18. Costanzo MR, Saltzberg M, O’sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol 2005, 46(11):2047-2051.
19. Costanzo MR, Saltzberg M, O’sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am CollCardiol 2005, 46(11):2047-2051.
20. Bartone C, Saghir S, Menon SG, et al. Comparison of ultrafiltration, nesiritide, and usual care in acute decompensated heart failure. Congest Heart Fail 14(6):298-301.