Abstract
With the ongoing pandemic of coronavirus disease 2019 (COVID-19), many neurological manifestations have been linked to what began as an outbreak of atypical pneumonia. Like other corona viruses, severe acute respiratory syndrome coronavirus (SARS-CoV-2) has potential for neurotropism. Viral invasion and likely pathogenecity has been associated with expression of ACE-2 receptors along endothelia and olfactory mucosa. Hyperinflammatory response after systemic infection is in part responsible for the severity and multi-organ dysfunction seen in severe cases. Features like encephalitis, central and peripheral demyelinating disorders, cranial neuropathies, stroke are seen not only in patients with severe illness but at times preceding any systemic symptoms. Neurological disorders are not only part of acute illness phase but also seen in postinfectious phase likely due to immune mediated effect. Also, long term sequelae in patients with moderate to severe COVID-19, either due to systemic complications or direct virus mediated effect are a matter of concern. High suspicion and early recognition of possible presenting features and neurological complications in severely affected patients is key for management. With the rapidity and enormity of ongoing pandemic whether these observations are causal or casual is yet to be determined. Further collaborations for comparisons of observations across the world in necessary to understand the potential impact of COVID-19. Accessing these observations will help in better understanding the agent host and environmental factors determining the expected impact of this pandemic. Reorganizing health care facilities to apt for better handling COVID-19 over time to come will help to mitigate the impact.
Keywords: COVID-19; neurological manifestations; encephalitis; stroke; neurotropism
Full Text
Background
What began as an outbreak of atypical pneumonia at Wuhan, China in November 2019 soon spread worldwide causing coronavirus disease 2019 (COVID-19) being declared as pandemic by World Health Organisation on March 11, 2020 [1]. With the spreading pandemic the knowledge of spectrum of COVID-19 is still evolving. Although pulmonary complications are characteristic, novel clinical data is emerging on extra-pulmonary especially neurological associations in COVID-19, expanding the gamut of this entity and further adding to the complexity of the pathogenicity. Neurological disorders not only add to the morbidity and mortality of the illness but at times may be the sole manifestations without any systemic features thereby misleading the diagnosis and possibly under-reporting.
SARS-CoV-2 and Potential for neuro-tropism
The severe acute respiratory syndrome coronavirus (SARS-CoV-2) (causing COVID-19) along with MERS-CoV (causing Middle East respiratory syndrome outbreak in 2012) and SARS-CoV-1 (causing severe acute respiratory syndrome outbreak in 2002-2003) belong to β-genus of the coronavirinae sub-family and share homologous genomic sequences [2]. SARS-CoV-2 is an enveloped, non-segmented, single stranded, positive sense RNA virus. There are three main viral proteins in the viral envelope: S- the spike protein, M- the membrane protein and E- the envelope protein. Both SARS-CoV-1 and 2 use the Spike protein to bind to the Angiotensin converting Enzyme 2 (ACE2) receptors on host cells. The presence of these ACE2 receptors determines the cellular tropism of SARS-CoV-2 in humans [3]. These ACE2 receptors are expressed in airway epithelia, renal cells, small intestine, vascular endothelia and widely throughout the nervous system. In the human brain these receptors are densely localised in substantia nigra, ventricles, middle temporal gyrus olfactory bulb and posterior cingulate gyrus. Within these tissues they are expressed by the neuronal cells, astrocytes And the oligodendrocytes [4].
Modes of neuroinvasion
The potential ways virus may invade nervous system is still unclear. One mechanism is trans-synaptic retrograde neuronal transfer through the olfactory bulb. The sustentacular and stem cells in the olfactory mucosa express ACE2 receptors and are vulnerable for SARS-CoV2 infection. Trans-synaptic transfer using endo- or exo-cytosis and vesicular axonal transport is proposed for retrograde spread from olfactory epithelia to olfactory bulbs within the central nervous system [5]. Endothelial cells across the cerebral vasculature express ACE2 receptors. Slow movement in cerebral microvasculature may further promote interaction with SARS-CoV-2 which can infect, damage and bud from capillary endothelia [6]. Infected leukocytes including T-lymphocytes, monocytes permeate through blood brain barrier during systemic inflammatory response and may act as a portal for entry into the nervous system. This process has been termed as Trojan horse mechanism [7] (Figure 1).
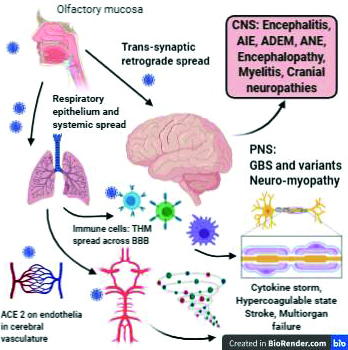
Figure 1: Neuroinvasion and neuropathogenesis of SARS-CoV-2 and clinical manifestations.
ACE2: Angiotensin converting enzyme receptor 2, Immune cells: T-cells, macrophages, B-cells, THM: Trojan horse mechanism, CNS: central nervous system, PNS: peripheral nervous system, AIE: autoimmune encephalitis, ADEM: acute disseminated encephalomyelitis, ANE: acute necrotizing encephalitis, GBS: Guillaine Barré syndrome.
Role of systemic complications
Although potential for direct neurological stigmata by SARS-CoV-2 has been thus implicated, the clinical picture is often complicated by systemic factors especially in severely ill patients and those with co-morbidities.
Coagulopathy
Coagulopathies are commonly observed in COVID-19 and are marker for poor outcome. Elevated prothrombin time, raised D-dimer levels, thrombocytopenia with or without hypofibrinogenemia are markers of COVID-19 associated coagulopathy (CAC). Virus mediated downregulation of ACE2 receptors with concurrent activation of Renin-Angiotensin system on one side and profound systemic inflammatory response on the other contribute to stroke and other pro-thrombotic events [8].
Cytokine storm
Immune system responds to any pathogen by activation of inflammation cascade with release of cytokines. SARS-CoV-2 at times may result in hyperactive immune response and massive release of pro-inflammatory cytokines like IL-6, IL-1, IL-8, IL-17 and TNF-α. This in turn leads to influx of immune cells including macrophages, neutrophils, T-cells and consequent damage to vascular barriers, capillary damage, endothelial destabilisation, coagulopathy with multi-organ failure. Managing such severely ill patients requires prolonged ICU care and portends long term consequences [9].
Autoimmunity
Apart from the above mentioned hyper-inflammatory response with cytokine storm, COVID-19 has been associated with Guillaine barre syndrome (discussed in subsequent sections), Miller fischer syndrome, APLA syndrome [10, 11], pediatric Kawasaki like disease [12, 13], probable immune thrombocytopenias. SARS-CoV-2 may act as trigger for such hyper-inflammatory or autoimmune response either as a superantigen response with clonal T cell proliferation and massive release of cytokines [14]. Auto-antibody formation by molecular mimicry may be a possible mechanism apart from immune complex mediated vascular damage. Lack of uniformity in evaluation with insufficient data on role of antibody measurement, absence of typical respiratory symptoms in few cases at presentation may impede understanding the pathogenesis and therefore merits further research.
Neuropathology
With evolving literature, pathological lesions likely responsible for the observed neurological manifestations are being described paving way for better understanding the neuropathogenesis of SARS-CoV-2 [15]. Lesions include vascular and demyelinating characteristics such as myelin loss, macrophage clusters, perivascular inflammatory infiltrate with axonal injury as well as sometimes nonspecific findings or no abnormalities at all in sampled brain tissue. Thus the possible mechanism for neuronal injury are still a matter of debate as direct virus induced toxic effect or parainfectious inflammatory result or a combination of both.
Neurological manifestations
SARS-CoV-2 spreads predominantly from respiratory droplets from symptomatic and asymptomatic carriers. It commonly manifests with fever and dry cough, although gastro-intestinal symptoms and rhinorrhoea has also been reported [16]. With growing literature and methodological differences it is difficult to generalise the results and estimate exact prevalence of neurological syndromes. The first case series from Wuhan, china reported almost 36.4% proportion of neurological symptoms with COVID-19 [17]. However the Spain ALBACOVID registry reported neurological symptoms in 57.4% [18].
Although neurological manifestations are not as commonly observed given the scale of current pandemic and the potential of transmission, the future implications and long term consequences are uncertain. Neurological manifestations may result either by direct effect of the virus on the nervous tissue and/or indirectly via immune system activation or from the effect of systemic complications of other organ systems involved. The direct effects may be noted in acute phase of the illness manifesting as encephalitis, toxic encephalopathy while the indirect effects present late after days to weeks after the initial viral prodrome. These effects have also been noticed without any obvious prior acute illnesses. Systemic complications may portend to hypoxic or critical illness related neurological complications.
Central nervous system
Meningo-encephalitis
After an early case report of a young man with viral meningitis with fever and generalised seizure was reported from Japan [19], several case reports were published with encephalitis presentation with COVID-19. A multicentre study from 13 centres in Italy described 25 cases with encephalitis associated with SARS-CoV-2 (Preprint not yet peer reviewed) [20]. Among these delirium (68%), speech disturbances (24%) and seizures (24%) were the most common presenting symptoms. The neurological symptoms were concomitant in 44%, following systemic COVID19 symptoms in 48% while in rest preceding systemic symptoms by 3-5 days. All cases were confirmed with throat or naso-pharyngeal swab for SARS-CoV-2 RT PCR, CSF samples were negative for PCR. MRI showed features of ADEM (acute disseminated encephalomyelitis), Limbic encephalitis (LE), non-specific MRI alterations while normal imaging in some cases. Treatment included high dose methylprednisolone, IVIg and plasmapheresis. ADEM and LE cases showed delayed onset and poor treatment response and had more severe respiratory involvement.
Acute necrotizing encephalopathy (ANE)
There are reports of acute necrotizing encephalitis like presentation with COVID-19. One case had prior viral prodrome, showed bilateral thalamic hemorrhagic lesions and received IVIg, outcome was not reported [21]. Other had no preceding systemic features, presented with severe encephalopathy, progressing to catatonia like syndrome, had bilateral thalamic and hippocampal hyperintensities, received methylprednisolone with IVIg and rituximab 1gm infusion following which she recovered and was discharged to rehabilitation centre [22]. Although rare, this entity caries major risk of significant disability and mortality, is presumed to be post or para-infectious hypercytokinemia related disruption of blood brain barrier and subsequent neuronal injury.
Acute disseminated encephalomyelitis (ADEM)
ADEM is a usually monophasic immune mediated demyelinating disorder preceded by febrile illness or recent vaccination. It presents with encephalopathy, multi-focal deficits, seizures. ADEM associated with COVID-19 has been reported may also be linked to cellular or humoral immunity against Myelin oligodendrocyte glycoprotein (MOG) [23]. A 56yrs woman with severe respiratory COVID-19 developed severe encephalopathy with quadriplegia after extubation, MRI revealed bilateral periventricular white matter hyperintensities consistent with ADEM, showed clinical recovery with intravenous methylprednisolone with Tocilizumab [22]. UK based study [24] reported about 9 cases with ADEM associated with COVID 19. Of these 5 had hemorrhagic encephalitis one had concurrent polyradiculopathy, one underwent decompression. Six patients received MPS, one dexamethasone. One succumbed to the illness while 3 recovered and rest were ongoing therapy by the time the data was published. Although the exact pathophysiology of direct neuronal injury versus inflammatory response still is a matter of debate, above cases should alert clinicians for possible ADEM like presentation or evolution in already sick COVID-19 patients.
New onset seizures, status epilepticus
New onset seizures as presenting feature in COVID-19 have been reported. One case reported had coexisting comorbidities and multi-system involvement with severe COVID-19 status and likely symptomatic seizures evolving to status epilepticus [25]. A retrospective case series of seven patients presenting with seizures and diagnosed with COVID-19 was reported [26]. Out of the seven patients four had new onset seizures while 3 had breakthrough seizures with prior well controlled epilepsy. While viral illnesses are known risk factor for precipitating seizures, complicated with systemic metabolic derangements and inflammatory response, early evaluation for likely association with CoV-2 may help in timely care and management.
Autoimmune encephalitis
There are 2 cases reported with new onset refractory status epilepticus with psychosis due to anti-NMDA receptor encephalitis and concomitant COVID-19 [27, 28]. Both patients presented solely with behavioural disturbances and later seizures evolving to status epilepticus, one had no respiratory symptoms at all, while other developed respiratory complications after admission. With the outburst of cases with COVID-19, whether this association was casual or causal is still a matter of debate. While role of Herpes simplex to trigger genesis of autoimmune particularly Anti-NMDA encephalitis is well known, in both the above mentioned cases development of neurological syndromes was concomitant with COVID-19. Given the potential to trigger profound immunological response with cytokine storm, the likelihood of SARS-CoV-2 to trigger autoimmunity may not be completely excluded as a chance association. Furthermore, implications of the immunomodulatory treatments used in treatment of autoimmune encephalitis on SARS-CoV-2 and the inflammatory cascade it triggers are yet to be understood.
Encephalopathy
Encephalopathy or alteration in sensorium is usually multi-factorial. It may present most commonly as mere drowsiness, confusion, agitation, executive or cognitive disturbances. Especially in moderate to severely ill patients with COVID-19 with multiorgan dysfunction wide range of causes may be contributory including hypoxia, metabolic derangements, medications and prolonged ICU stay. The proportion of patients with encephalopathy reported in various studies differs markedly from 7-69% [29]. But elderly, those with pre-existing comorbidities, severe illness are at higher risk and indicate poor outcome [17].
Myelitis
Occurrence of acute transverse myelitis in association with COVID-19 has very well been reported. The onset of neurological symptoms ranges from 2 days to 2 weeks following initial mild to moderate respiratory illness [30-33]. In few cases there were no preceding or concurrent respiratory symptoms [31]. Like the aforementioned entities, onset of myelitis as a direct viral mediated infective or inflammatory process or post-infectious autoimmune reaction needs to be elucidated. SARS-CoV-2 was tested typically in the nasal swab in almost all cases, showing negative PCR in CSF [33], and one [32] showing positive serology with both IgM and IgG at day 20 of illness. All cases showed improvement with IV MPS except one [33] which had long segment cervical cord involvement and had significant residual disability despite plasmapheresis.
Cerebrovascular events
Stroke has been described widely in patients with COVID-19.Interaction between conventional vascular risk factors and virus mediated endothelial destabilisation, systemic inflammatory response and disruption of ACE2 mediated vascular autoregulation plays complicated role. Concurrent systemic multi-organ involvement in the form of myocarditis, procoagulant state may pose risk of thrombo-embolic events. Few case series and vast number of case reports have shown occurrence of stroke in COVID-19 [34-38]. Comparison of clinical parameters in few series is shown in Table 1. Potential mechanisms mediating occurrence of stroke in COVID-19 are illustrated in Figure 2 [39].
Table 1: Clinical characteristics of Stroke associated with COVID-19.
|
Study
|
Country
|
No. of patients
|
Mean age (range) and sex (M)
|
Days from systemic COVID19 features
|
Type of stroke
|
Vascular risk factors
|
Biochemistry
|
Treatment given
|
|
Beyrouti et al., [34]
|
UK
|
6: infarcts
|
69.8 years (53-85)
M=5
|
11.6 days (-2 to 24days)
|
Ishemic large vessel occlusion
|
HTN-4
DM-2
Ca: 2
AF: 2
CAD:2
None:1
|
D-dimer >7000 mcg/L,
LA+:5
Ferritin and LDH ↑↑
|
Thrombolysis in 2
LMWH: 3
Apixaban:1
(one died, rest details NA)
|
|
Li Y. et al., [35]
|
China
|
11: Infacrts
1: ICH
1:CVT
|
75 years (57-91)
M=8
|
10 days (1-29)
|
5: large vessel occlusion
2: small vessel
3: cardioembolic
|
HTN: 7
DM:8
CAD:3
Ca:1
|
D-dimer: 6000mcg/L
|
SAPT: 6
LMWH: 4
|
|
Oxley et al., [36]
|
USA
|
5: infarcts
|
40.4 years (33-49)
M:4
|
3 had no systemic symptoms, 2 had concurrent viral prodrome
|
5: large vessel occlusion
|
HTN:1,
DM:2
Old CVA:1
Dyslipidemia:1
None: 2
|
D-dimer: elevated in 3(normal in 2)
|
EVT: 3
Apixaban:2
SAPT:1
DAPT:1
|
|
Khan et al., [37]
|
UAE
|
22: infarcts
|
46.3 years
M: 20
|
18: stroke at presentation
12: preceding respiratory smptoms: 1-7days
4: in hospital strokes 4-21days
|
22: large vessel occlusion: 13: anterior
6: posterior
3: multiple territories
|
DM:8
HTN: 7
CAD: 2
Dyslipidemia: 2
Old CVA: 1
|
D-dimer: ↑in 81.8%
Ferritin: ↑ in 58.8%
|
2: IV TPA,
EVT: 2
8: LMWH
14: antiplatelet
6: died
|
|
Immovilli et al., [38]
|
Italy
|
19:
17: infarcts
2: ICH
|
76 years
M=10
|
15: systemic symptoms preceding stroke
4: stroke 2.5 days preceding
|
4: large vessel occlusion
5: cardio-embolic
2: small vessel disease
6: undetermined
|
DM: 10.5%
HTN: 84.2%
AF:31.6%
|
NA
|
3: IVtPA
|
M: Male, HTN: Hypertension, DM: Diabetes mellitus, Ca: Solid organ carcinoma, CAD: Coronary artery disease, AF: Atrial fibrillation, LA: Lupus anticoagulant, NA: Not available, SAPT: Single antiplatelet aspirin or clopidogrel, DAPT: Dual antiplatelet agents.

Figure 2: Potential mechanisms underlying occurrence of stroke in COVID-19.
DIC: Disseminated intravascular coagulation, RAS: Rennin angiotensin system, HTN: Hypertension, DM: Diabetes mellitus, CAD: Coronary artery disease, AF: Atrial fibrillation
Management of stroke in COVID-19 patients is particularly challenging. With progressively increasing case load of all patients, screening for COVID-19 related symptoms clinically as well as radiologic investigations may delay addressing stroke especially those in window period for intervention. Technical delays in transport further makes seeking timely medical attention difficult. Although principles in managing stroke remain same as in non-COVID patients, yet the decisions have to be individualised based on severity of systemic illness, co-morbidities [40]. Further evaluation on use of prophylactic anti-coagulation in moderate to severely sick COVID-19 patients and stroke prevention is awaited.
Peripheral nervous system
Guillain-Barré syndrome (GBS) and variants
Inflammatory polyradiculoneuropathy is known to occur following prior infective prodromes. A review [41] of around 37 patients with GBS with COVID-19 from 28 publications showed mean age of 59years (90% above 50-years-old) and 65% of all being males. Two patients presented with neurological symptoms. Both had prior contact but no systemic features although showed ground glass opacities on CT chest at presentation suggesting asymptomatic infection. Rest of the cases had mean time interval of 11 ± 6.5 days (range, 3-28 days) from onset of systemic symptoms of COVID-19. Around 31 of 37 patients had developed GBS features while ongoing symptoms with COVID-19. Although clinical characteristics were similar to non-COVID GBS cases, over half of them had demyelinating features on neurophysiological evaluation. About 76% patients had albumin-cytological dissociation while all cases were negative for CSF RT-PCR for SARS-CoV-2. Most patients received single course of IVIg and showed clinical improvement over 8 weeks post illness. Respiratory involvement is more severe and may result from respiratory muscle paralysis along with interstitial pneumonia due to COVID-19. Further study on temporal evolution of GBS and serological correlation with immune response to COVID-19 is warranted.
Occurrence of Miller Fischer syndrome characterised with ataxia, ophthalmoplegia and areflexia and polyneuritis cranialis has been reported with COVID-19 [42]. Both patients had recovery following treatment with IVIg.
Myositis and rhabdomyolysis
Either as a part of critical illness myopathy-neuropathy or immune mediated skeletal muscle injury might underlie elevated creatinine kinase levels seen in COVID-19. Severe illness, metabolic derangements and drugs used in treating COVID-19 may contribute to myopathy [17].
Miscellaneous symptoms
Although commonly reported non-specific symptoms including headache, fatigue, myalgia [17] may portend more of systemic illness. General fatigue, headache with myalgias is commonly reported early feature in COVID-19 [29].
Anosmia, dysguesia
Altered sense of smell and taste or more likely loss of these senses has been associated with COVID-19. In the first mentioned series [17] hypoguesia was reported in 5.6% while hyposmia in 5.1% cases. Later however a multicentre study from 12 European hospitals reported that olfactory and gustatory dysfunction was present in 85.6% and 88.0% of lab confirmed COVID-19 cases, and females were significantly more likely to be affected than males. Olfactory dysfunction preceded other systemic symptoms in 11.8% [43]. Direct local viral invasion mediated through ACE2 receptors may play role in both sensory dysfunctions. Difference in expression of ACE2 receptors may by underlying different response and susceptibility to SARS-CoV-2 [44].
Psychiatric, cognitive sequelae
Preceding, concurrent or post-infectious neurological manifestations associated with COVID-19 have been well described. Concern is also raised for possible long term sequelae particularly in terms of cognitive decline [45]. This may be seen in prolonged ventillatory care and ICU stay in severely ill patients. Hypoxia, systemic complications, microvascular disturbances and aggravation of pre-existing cognitive deficits in elderly may be responsible. Although anosmia and dysgeusia may be transient and tend to recover over the time, likelihood of developing Parkinsonian Syndromes either secondary to systemic complications or direct neurotoxicity of the virus mediated through the ACE2 receptors has been proposed [46]. These findings are still under review and may only be determined on long term follow up of COVID-19 cases as the pandemic evolves.
Possible neurological effects of therapies for COVID-19
Chloroquine and hydroxychloroquine (HCQ) act by preventing viral entry by endocytosis through ACE2 receptors. Although due to potential cardiac adverse effects US FDA recommends exercising caution in use of these drugs. Neurological symptoms in the form of peripheral neuropathy, neuromyopathy, muscle cramps, rhabdomyolysis, irritability and psychosis are known to occur with these drugs. Also HCQ can lower seizure threshold, interacts with anti-seizure medications including lacosamide and lamotrigine and may precipitate myasthenia gravis [3].
Potential interactions between commonly used anti-viral drugs including remdesivir and anti-epileptic drugs need to be monitored. Anti-inflammatory therapies with anti-IL6 monoclonal antibody Tocilizumab has been associated with acute neurodeficits due to multifocal cerebral microangiopathy [47]. When evaluating cases with neurological deterioration in COVID-19 possibility of medication related adverse events also need to be considered as well.
Conclusion
Although proportion of neurological manifestations associated with COVID-19 is small, considering the continuing pandemic and prediction of nearly 50-80% of world population being affected before herd immunity develops, the predicted numbers of cases with neurological disorders would be alarming [48]. Stroke associated with hypercoagulant state or immune mediated encephalitides linked to COVID-19 may have long term morbidities. With extensive number of cases in growing pandemic the causal role of SARS-CoV-2 or incidental association in asymptomatic carriers merits further study. Scientific communities across the world are tied in race against time to develop treatment options and plausible vaccines for this global threat. Worldwide collaboration across centres catering care for COVID-19 should be encouraged for uniformity in reporting and comparability of novel observations linked to COVID-19 because desperate times call for desperate measures!
Conflicts of interest
Author declares no conflicts of interest.
References
[1] World Health Organization. Rolling updates on coronavirus disease (COVID‐19). Updated 24 April 2020. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐as‐they‐happen.
[2] Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019; 17(3):181–192.
[3] Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A Review. JAMA Neurol. 2020; 77(8):1018–1027.
[4] Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. bioRxiv; 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.04.07.030650v1
[5] Fodoulian L, Tuberosa J, Rossier D, Landis BN, Carleton A, et al. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. Published April 20, 2020. Accessed on 18 May 2020 from: https://www.biorxiv.org/content/10.1101/2020.03.31.013268v1
[6] Cardona CG, Pájaro QLD, Marzola QID, Villegas RY, Salazar MLR. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J Neurol Sci. 2020; 412:116824.
[7] Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, et al. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System?. Viruses. 2019; 12(1):14.
[8] Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, et al. Coronavirus disease 2019 and stroke: Clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020; 29(8):104941.
[9] Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020; 11:1446.
[10] Zhang Yan, Xiao Meng, Zhang Shulan, Peng Xia P, Cao W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020; 382(17):e38.
[11] Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020; Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1111/jth.14867
[12] Jiatong S, lanqin L, Wenjun L. COVID-19 epidemic: Disease characteristics in children. J Med Virol. 2020; 92(7):747-754.
[13] Carsetti R, Quintarelli C, Quinti I, Mortari EP, Zumla A, et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Health. 2020; 4(6):414-416.
[14] Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020; 19(8):102597.
[15] Reichard, R. R. et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020; 140:1–6.
[16] World Health Organization. Report of the WHO China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
[17] Mao L, Jin H, Wang M, Hu Y, Chen S, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6):683–690.
[18] Romero- Sánchez, C. M. et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020; 95(8):e1060–e1070.
[19] Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020; 94:55–58.
[20] Pilotto A, Masciocchi S, Volonghi I, Zotto E, Magni E, et al. The clinical spectrum of encephalitis in COVID-19 disease: The ENCOVID multicentre study. MedRxiv. 2020; Available from: https://www.medrxiv.org/content/10.1101/2020.06.19.20133991v1
[21] Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020; 201187.
[22] Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, et al. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020; 17(1):231.
[23] Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, et al.. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016; 87(9 Suppl 2):S38–45.
[24] Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, et al. The UCL Queen Square National Hospital for Neurology and Neurosurgery COVID-19 Study Group, The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020; 143(10):3104–3120.
[25] Sohal S, Mansur M. COVID-19 Presenting with Seizures. IDCases. 2020; 20:e00782.
[26] Anand P, Al-Faraj A, Sader E, Dashkoffa J, Abdennadher M, et al. Seizure as the presenting symptom of COVID-19: A retrospective case series. Epilepsy Behav. 2020; 112:107335.
[27] Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020; 81:18–20.
[28] Panariello A, Bassetti R, Radice A, Rossotti R, Puoti M. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immnunity. 2020; 87:179–181.
[29] Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020; 382(23):2268–2270.
[30] Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020; 13(8):e236720.
[31] AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, Samir M, et al. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol Case Rep. 2020; 15(9):1591–1595.
[32] Zachariadis A, Tulbu A, Strambo D, Dumoulin A, Virgilio GD, et al. Transverse myelitis related to COVID-19 infection. J Neurol. 2020; 267(12):3459–3461.
[33] Valiuddin H, Skwirsk B, Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: A Case-Report. Brain Behav Immun Health. 2020; 5:100091.
[34] Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020; 91(8):889–891.
[35] Li Y, Li M, Wang M, Zhou Y, Chang J, et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc Neurol. 2020; 5(3):000431.
[36] Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020; 382(20):e60.
[37] Khan M, Ibrahim RH, Siddiqi SA, Kerolos Y, Al-Kaylani MM, et al. COVID-19 and acute ischemic stroke - A case series from Dubai, UAE. Int J Stroke. 2020; 15(6):699–700.
[38] Immovilli P, Terracciano C, Zaino D, Marchesi E, Morelli N, et al. Stroke in COVID-19 patients-A case series from Italy. Int J Stroke. 2020; 15(6):701–702.
[39] Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020; 77(11):1–7.
[40] Bhatia R, Srivastava MVP. COVID-19 and Stroke: Incidental, triggered or causative. Ann Indian Acad Neurol. 2020; 23(3):318–324.
[41] Caress, JB, Castoro, RJ, Simmons, Z, et al. COVID‐19–associated Guillain‐Barré syndrome: The early pandemic experience. Muscle Nerve. 2020; 62(4):485–491.
[42] Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, Pedro-Murillo ES, Bermejo-Guerrero L, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020; 95(5):e601–e605.
[43] Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Bon SDL, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8):2251–2261.
[44] Cao Y, Li L, Feng Z, Wan S, Huang P, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020; 6:11.
[45] Heneka, MT, Golenbock D, Latz E, Morgan D, Brown R, et al. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020; 12(1):69.
[46] Pereira A. Long-term neurological threats of COVID-19: A call to update the thinking about the outcomes of the coronavirus pandemic. Front Neurol. 2020; 11:308.
[47] Jewell P, Ansorge O, Kuker W, Irani SR, Zamboni G. Tocilizumab-associated multifocal cerebral thrombotic microangiopathy. Neurol Clin Pract. 2016; 6(3):e24–e26.
[48] Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020; 194:105921.