Original Research
2014
June
Volume : 2
Issue : 2
Enhancement of paclitaxel and doxorubicin cytotoxicity in breast cancer cell lines in combination with piperine treatment and analysis of expression of autophagy and apoptosis genes
Pushpa Ragini S, Naga Divya AV, Anusha Ch, Kanthaiah YV
Pdf Page Numbers :- 62-67
Pushpa Ragini S1, Srinivasa Rao B1, Naga Divya AV1, Anusha Ch1 and Kanthaiah YV1,*
1KIMS Foundation and Research Centre, Minister Road, Secunderabad - 500003, AP., India.
*Corresponding author: Dr. YV. Kanthaiah, KIMS Foundation and Research Centre, Minister Road, Secunderabad - 500003, AP, India. Email: kantha10@yahoo.com
Received 10 February 2014; Revised 24 March 2014; Accepted 31 March 2014
Citation: Pushpa Ragini S, Naga Divya AV, Anusha Ch, Kanthaiah YV (2014) Enhancement of paclitaxel and doxorubicin cytotoxicity in breast cancer cell lines in combination with piperine treatment and analysis of expression of autophagy and apoptosis genes. J Med Sci Res 2(2):62-67. DOI: http://dx.doi.org/10.17727/JMSR.2014/2-012
Copyright: © 2014 Pushpa Ragini S et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Breast cancer treatment failure mainly attributed to drug resistance and suboptimal dosage of drugs reaching to the actual target. Several Bioenhancers have been used for enhancing drug availability to the target cancer cells in sustainable manner. Piperine is one such molecule has been used for increasing serum drug concentrations by enhancing the drug absorption in the stomach. However it is not clear whether piperine will have direct effect on cells when used along with drugs. In the present study we have studied the direct effect of piperine in combination with Doxorubicin and paclitaxel.
Methodology: MDA MB -231 cell lines were cultured and treated with different concentrations of paclitaxel and Doxorubicin. The piperine at 25 µM was added to all drug concentrations. Induction of cytotoxic effect of doxorubicin and paclitaxel in combination with piperine was analysed by MTT cytotoxic assay, CFSE cell proliferation assay. Further the apoptotic and autophagy genes i.e. Beclin 1, P21, Bax and Survivin expression were analysed by semi quantitative RT PCR.
Results: Piperine enhanced the cytotoxicity effect of doxorubicin and paclitaxel at all concentrations of drugs. Piperine alone did not cause cytotoxicity or inhibition of cell proliferation. However piperine enhanced the cytotoxic and anti proliferative effect of paclitaxel and doxorubicin when used in combination. Further, piperine in combination with drugs shown to induce P21 expression and reduce surviving expression. We have not observed changes in Beclin 1 and Bax genes expression with either drugs alone or in piperine combination.
Conclusion: Piperine has increased the cytotoxic and anti proliferative affects of doxorubicin and Paclitaxel drugs when used directly in cell lines. However the molecular mechanism has to be further analysed to understand the actual mode of action of piperine in breast cancer cells.
Keywords: Breast cancer; Piperine; MDA MB -231; Paclitaxel; Doxorubicin
Full Text
Introduction
Breast cancer is a highly heterogeneous disease. Different types of this neoplasm exhibit variable histopathological and biological features, different clinical outcome and different response to systemic interventions. Drug resistance is a major limitation in breast cancer chemotherapy and frequently accounts for the failure of chemotherapy. It exists in two forms: acquired resistance, where the drug is initially efficient but becomes ineffective over time, while intrinsic resistance occurs when a drug is ineffective from the beginning of the treatment. Generally, the following mechanisms could be involved in chemo-resistance: (i) Decreased intracellular drug concentrations (drug transporters, metabolic enzymes) (ii) Resistance to cell death due to disturbances affecting the cell cycle arrest, apoptosis and DNA repair (iii) Activation of signaling pathways related to the progression of cancer (iv) Epigenetic modifications and (v) Alterations in the availability of drug at the targets [1].
Though the molecular mechanism is unknown, in order to overcome drug resistance most of the times combinations of anticancer drugs are used in chemotherapy regimens. It is also proved that chemotherapeutics when given with natural bioenhancers are able to overcome acquired drug resistance. Bioenhancers are the molecules which by themselves do not show typical drug activity, but when used in combination enhance the activity of drug molecules in several ways without modifying its activity. Efficacy drug depends upon its bioavailability which in turn depends upon the rate at which the unchanged drugs are made available to the body and the extent to which the dose is ultimately absorbed after administration. Bioenhancers are considered to enhance the bioavailability of companion drugs either by inhibiting the drug metabolizing enzyme, cytochrome P450 [2] or by inducing the drug activity by interfering with drug resistance mechanisms. In the present study we investigated whether natural compound piperine acts as an enhancer of the drug cytotoxicity. Piperine is a major alkaloid of Pepper fruits belonging to family Piperaceae. It is endowed with a number of medicinal properties [3]. Piperine enhances the bioavailability of structurally and therapeutically different drugs, either by increasing the absorption or by delaying the metabolism of the drug or by a combination of both processes. Reen and Singh considered that piperine increases the absorption of drugs from gastrointestinal tract by causing direct effect on vascular endothelium, smooth muscle and mast cells, resulting in increased vascular permeability and mucosal blood flow [2]. Piperine was observed to decrease the TER and thus increase the pore size between the cells and in turn the permeability of the intestinal milieu resulting in higher rate and extent of absorption of different drugs [4]. Piperine may also interact with the process of oxidative phosphorylation process like activation/ deactivation of certain metabolic pathways [5], slowing down the metabolism and biodegradation of drugs. This action of piperine results in higher plasma levels of the drugs, rendering them more available for pharmacological action. Atal et al. in 1981 mentioned that piperine enhanced the antiasthamatic property of vasaka leaves by increasing the bioavailability of vasicine [6], the active ingredient of vasaka leaves. Increased bioavailability of a number of drugs such as oxyphenbutazone [7], phenytoin [8], aflatoxin B1 [9], theophylline and propranolol, rifampicin [10], dapsone [11], curcumin [12], ciprofloxacin and phenobaritone, were reported when piperine was used with these drugs. Apart from acting as a bioenhancer, piperine was also shown to possess antioxidant, antidiaorrheal, antidepressant, antiplatelet, anti-inflammatory [13, 14], antihypertensive, hepatoprotective, antithyroid, antitumor activities. Madhuri Kakarala et.al., determined the effect of curcumin and piperine on Wnt signaling in well characterized breast cancer cell lines utilizing the TCF-Lef Topflash reporter system and found that curcumin and piperine separately, and in combination, inhibited breast stem cell self renewal but did not cause toxicity to differentiated cells [15]. Makhov et al., performed studies on a xenograft model of human CRPC and investigated the pharmacokinetic and anticancer effects of piperine when co-administered with docetaxel. Results from their studies demonstrated that treatment with piperine inhibited hepatic CYP3A4 activity in vivo which coincided with an increased area under the curve (AUC), half-life, and maximum plasma concentration of docetaxel, without resulting in an increase in docetaxel-mediated toxicities when administered in combination with piperine versus docetaxel alone [16]. Stohr JR et al. reported inhibition effects of piperine on oxygenase, p450 isoenzyme and cycloxygenase-1 expression may contribute to the antimetastatic qualities [17]. Hwang YP et al. reported the suppression of the expression of MMP-9 in tumor cells by piperine is through the inhibition of PKCα and ERK phosphorylation and reduction of NF-κB and AP-1 activation [18]. Li-hua LAI et al. found that piperine treatment down-regulated the expression of cyclin B1 in 4T1 cells in a dose-dependent manner [19].
Materials and methods
Culturing of MDA MB -231 cell lines: MDA MB 231 cell lines were gifted by Institute of pathology, ICMR Delhi. Cell lines were maintained in DMEM basal media containing 10% FBS and 1% Penicillin – streptomycin solution. Cell limes were incubated in 5% CO2 at 37°C.
Cytotoxicity assay (MTT assay): Cytotoxicity assay for MDA-MB 231 cells was performed at different concentrations of Paclitaxel and doxorubicin along with piperine treatments as per previous protocols. Briefly, Cells were cultured in 96 well plates and incubated with Doxorubicin containing serial dilutions from 20 µM – 0.156 µM, and Paclitaxel serial dilutions from 100 µM to 1.5 µM. Piperine non cytotoxic concentration was determined after titration and used at 25 µM. Piperine at 25 µM was added to all treatment wells and incubated for 48 hrs in 5% CO2 at 37°C before proceeding for MTT (Sigma Aldrich) assay for cytotoxicity. The cytotoxicity was analysed by MTT assay following manufacturer’s protocol.
CFSE Cell proliferation assay: CFSE (Carboxyfluorescein succinimidyl ester) was procured from Invitrogen and titrated for cell proliferation analysis. Cells were cultured in 12 well plates at 105 cells/ well and incubated with specified drugs. The concentration of Doxorubicin and paclitaxel used were 10 µM and 50 µM respectively and piperine was constant at 25 µM in all treatments. The minimum cytotoxic effect of piperine was derived from the titration analysis and used at 25 µM as a non toxic concentration.
Gene expression analysis: Beclin 1, Survivin, P21 and Bax molecules expression were analysed by semi quantitative Reverse transcriptase PCR after treatment with doxorubicin, paclitaxel along with piperine as mentioned previously. Briefly, MDA MB cells were cultured in 6 well plates and treated with Doxorubicin, Paclitaxel along with Piperine. Total RNA was isolated from drug treated cells and cDNA is prepared using Reverse transcriptase. The prepared cDNA is used for quantification of Beclin 1, Survivin, P21 and Bax using sequence specific primers for cDNA. The GAPDH expression is measured and normalized for all samples. The Relative quantification of above genes is measured and analyzed.
Results
Piperine effect on doxorubicin cytotoxicity: Piperine alone was ineffective at 25 µM concentration. We determined it as non toxic at this concentration and checked for its adjuvant activity in combination with doxorubicin. The assay revealed that piperine enhanced the cytotoxic effect of doxorubicin at all concentrations and shown synergistic activity (Figure 1). However, piperine did not change the minimum effective concentration of doxorubicin when used in combination.
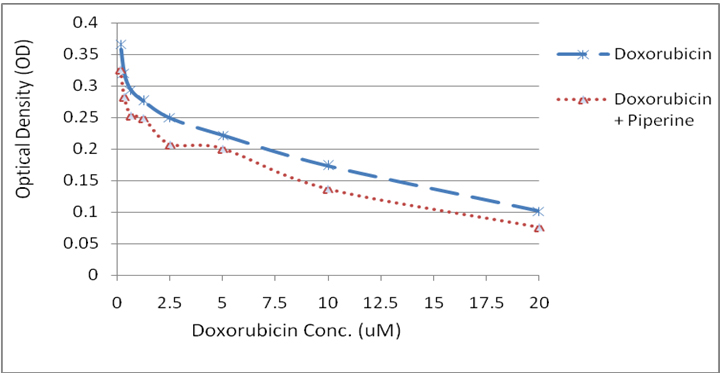
Figure 1: Graph showing the cytotoxic effect of doxorubicin at various concentrations (0.156 µM – 20 µM) and in combination with piperine.
Piperine effect on induction of paclitael cytotoxicity: The MTT assay revealed that piperine enhanced the cytotoxic effect of Paclitaxel at all concentrations and shown synergistic activity (Figure 2). Paclitaxel was cytotoxic at 50 µM dose and piperine has reduced the minimum effective concentration of paclitaxel to 25 µM when used in combination.
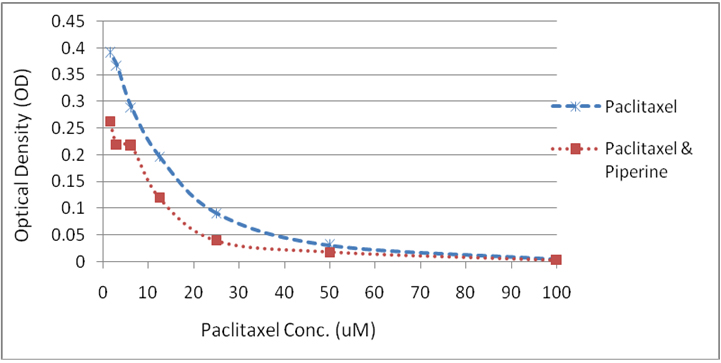
Figure 2: Graph showing the cytotoxic effect of Paclitaxel at various concentrations (0.15 µM – 100 µM) and in combination with piperine.
Effect of Doxorubicin and piperine in proliferation of breast cancer cells: Flowcytometry analysis showed that Doxorubicin effectively inhibited the cell proliferation showing maximum number of cells aggregated in generation 4 (38.46%) as compared to experimental control which shows maximum number of cells (26.90%) in generation 5. This indicates that cell proliferation was stalled in 4th generation with doxorubicin treatment. Further the number of cells remaining after doxorubicin treatment was considered alive and resistant to drug. Further, piperine in combination with doxorubicin affected cell proliferation evidenced by aggregating maximum cells in 4th generation and passed only 4.52% (Figure 4A) cells to 5th generation, where as in doxorubicin it was 10.60% (Figure 4B) .
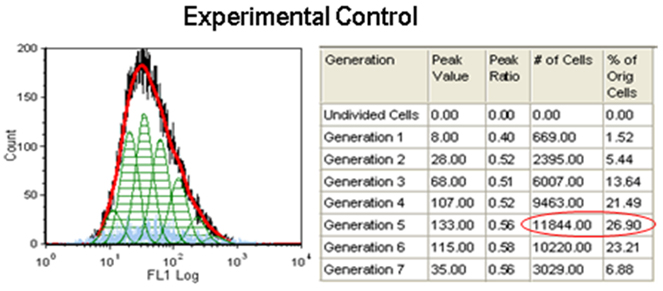
Figure 3: Experimental control for CFSE proliferation assay. Cells were added with CFSE and cultured for 48hrs. without any treatment.
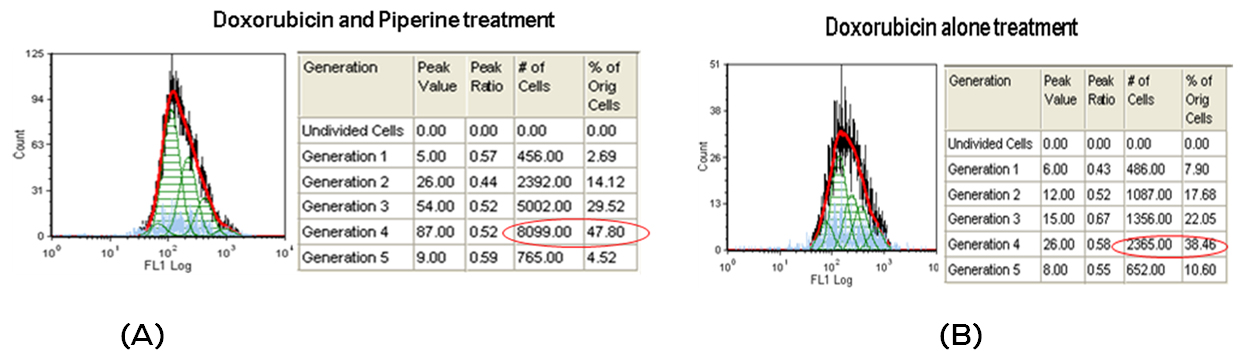
Figure 4: Flow diagrams for CFSE proliferation assay. A. MDA MB -231 cells treated with doxorubicine alone for 48hrs. and B. Cells treated with doxorubicin and Piperine.
Effect of Paclitaxel and piperine in proliferation of breast cancer cells: Flowcytometry analysis showed that paclitaxel effectively inhibited the cell proliferation showing maximum number of cells aggregated in generation 4 (48.63%) as compared to experimental control which shows maximum number of cells in generation 5 and 6. This indicates that cell proliferation was stalled in 4th generation with Paclitaxel treatment. Further the number of cells remaining after paclitaxel treatment was considered alive and resistant to drug. Further, piperine in combination with Paclitaxel affected cell proliferation evidenced by aggregating maximum cells in 3rd generation. As compared to doxorubicin, paclitaxel in combination with piperine is effective in inhibition of cell proliferation.
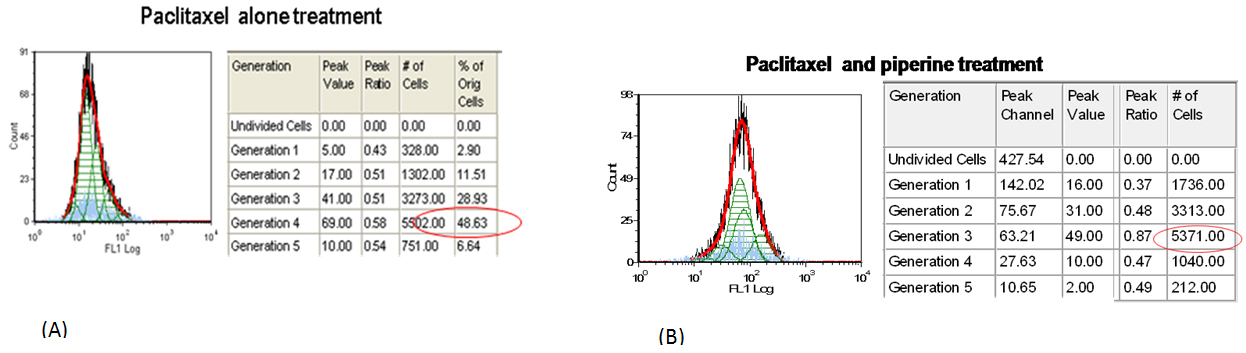
Figure 5: Flow diagrams for CFSE proliferation assay. A. MDA MB -231 cells treated with paclitaxel alone for 48hrs. and B. Cells treated with paclitaxel and Piperine.
Effect of paclitaxel and Piperine on Autophagy and apoptotic genes expression
Semiquntiative RT PCR was performed for analyzing the effect of paclitaxel and piperine combination treatments in the expression of Beclin 1, Survivin, P21 and Bax genes. GAPDH housekeeping gene expression was normalized to compare with the expression of above genes relatively. The semiquantitative RT PCR analysis showed that paclitaxel and in combination with Piperine has induced the P21 and down regulated the Survivin expression. However, Beclin 1 and Bax gene expression was not affected by any of the treatment.
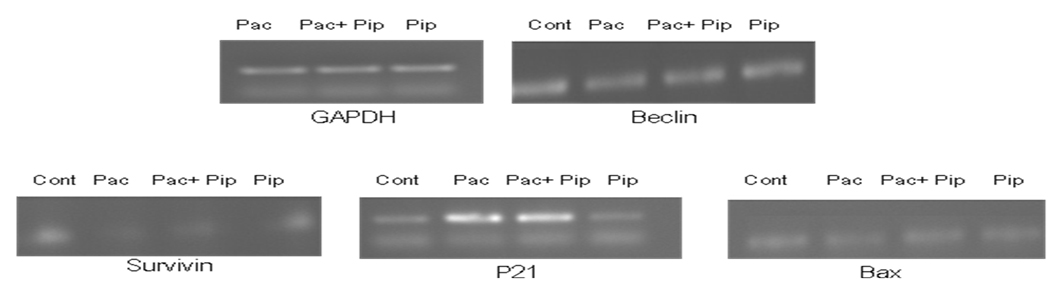
Figure 6: Semiquantitative RT PCR analysis of Beclin 1, Survivin, P21 and Bax genes after paclitaxel, Paclitaxel and piperine and only piperine combinations.
Effect of paclitaxel and Piperine on Apoptosis controlling genes expression
From the figures above survivin levels were found to be less compared to the expression levels of the other genes selected. Paclitaxel was found to have inducing effect on the expression levels of p21 and suppressing effect on the expression levels of survivin both in monotherapy and combination treatment whereas piperine was found to have no effect on apoptotic genes. Neither piperine nor paclitaxel treatment has shown effect on Bax levels.
Discussion
Paclitaxel and doxorubicin are widely used in clinics for the treatment of broad spectrum of cancers. Drug resistance and side effects are two major limitations that restricted their clinical success. Hence, active research is been carrying out on natural bioenhancers in combination with anticancer drugs as they do not exert any action by themselves but only modulates the action of anticancer drug. Recent studies by two different groups [20, 21] suggested the use of piperine in anticancer treatment. Hence, we demonstrated in this study the role of piperine in enhancing cytotoxic activity of anticancer drugs doxorubicin and paclitaxel.
Analysis of cytotoxicity assay revealed that piperine enhanced the cytotoxic activity of doxorubicin at all concentrations but no changes were observed in the minimum effective concentration of doxorubicin when administered in combination with piperine. Studies by different groups reported that doxorubicin induces cell death through apoptotic pathway [22, 23]. Tomoki Yokochi et al. reported through their studies that micromolar concentration of doxorubicin induces cell death through apoptotic pathway [24]. Cell proliferation assay results showed that doxorubicin stalled cell proliferation at 4th generation when used alone as well as combination with piperine but the number of cells passed to the next generation when doxorubicin alone was used is more when compared to the number of cells passed to the next generation when doxorubicin is used in combination with piperine. Studies by Young-Woo Eom et al. demonstrated that NF-kB, which is a potent regulator of antiapoptotic genes, is transiently activated during the initial phase of doxorubicin-induced apoptosis [23]. NF-kB dependent activation of prosurvival gene transcription will block apoptosis [25]. Piperine was proved to be a potent inhibitor of NF-κB [25, 19] and from this it can be assumed that when given in combination with doxorubicin, piperine might have enhanced its cytotoxicty activity.
Paclitaxel exerts antiproliferative actions by disrupting microtubule structure and function, resulting in the inability of cells to properly complete mitosis [26]. It was also found to induce cell death through apoptosis [27]. Cytotoxicity assay of paclitaxel revealed that paclitaxel when combined with piperine, its IC50 values were brought down from 50μM (IC50 value with paclitaxel alone) to 25μM. Decreased IC50 values are indicative of increased cellular concentration of drug. According to the findings of Ardith W. El-Kareh et al, peak total intracellular concentration to be a good predictor of drug activity [28]. This suggests that piperine might have increased the intracellular concentrations of paclitaxel either by facilitating increased cellular uptake of paclitaxel or by increasing the free, unbound form of paclitaxel inside the cell by disrupting its interactions with binding sites interfering with its intracellular pharmacodynamics. Analysis of cell proliferation assay results revealed that paclitaxel stalled cell proliferation at 4th generation whereas combination of piperine and paclitaxel stalled cell proliferation at 3rd generation. It suggests that piperine decreased the lag phase (which is attributed to the cell cycle specificity of paclitaxel) in the duration of action of paclitaxel which is consistent with the observations of Li-hua Lai et al that piperine treatment down-regulated the expression of cyclin B1, thus increasing the percentage of cells in G2/M phase in 4T1 cells [19].
In this study further investigated whether piperine exerts any influence on expression levels of autophagy and apoptosis controlling genes. Paclitaxel was found to have inducing effect on the expression levels of p21 which is in crrelationn with earlier studies of different groups [29, 30]. We also reported that paclitaxel decreased expression levels of survivin. P21 levels were induced to the same extent in both paclitaxel treated and paclitaxel, piperine combination treated cells. It is also reported that piperine alone has no influence on either autophagy or apoptosis controlling genes.
Conclusion
Combination treatment with piperine and paclitaxel yielded better antiproliferative and cytotoxic activity when compared to paclitaxel treatment alone. This combination is advantageous when compared to combination regimen which employs two or three cytotoxic drugs because piperine itself exerts no side effects and also because it increases cellular concentrations of paclitaxel dose escalating can be reduced.
Conflict of interest
The authors wish to express that they have no conflict of interest.
References
1. Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011; 3(1):1351-1371.
2. Reen RK, Singh J. In vitro and in vivo inhibition of pulmonary cytochrome P450 activities by piperine, a major ingredient of piper species. Indian J Exp Biol. 1991; 29(6):568-573.
3. Singh A, Deep A, “Piperine: A Bioenhancer” International Journal of Pharmacy Research and Technology 2011, Volume 1, Issue 1, 01-05.
4. Bhise SB, Pore VY, “Influence of co-administration of piperine on pharmacokinetic profile of ciprofloxacin,” Indian Drugs 2002; 39:166-168.
5. Majeed, Prakash L. The medicinal uses of pepper. International Pepper News 2000; 25(1):23-31.
6. Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985; 232(1):258-262.
7. Deshmukh VK, Dhuley JN, Naik SR, Mu-jumdar AM. “Effect of Piperidine on Bioavailability of oxyphenyl butazone in rats,” Indian Drugs 1999; 36:123-125.
8. Bano G, Amla V, Raina RK, Zutshi U, Chopra CL. The effect of piperine on pharmacokinetics of phenytoin in healthy volunteers. Planta Med. 1987; 53(6):568-569.
9. Allameh A, Saxena M, Biswas G, Raj HG, Singh J, et al. Piperine, a plant alkaloid of the piper species, enhances the bioavailability of aflatoxin B1 in rat tissues. Cancer Letters 1992; 61:195-199.
10. Zutshi RK, Singh R, Zutshi U, Johri RK, Atal CK. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. Journal of Association of Physicians India 1984; 33:223-224.
11. Singh A, Sharma SC, Zutshi U, Bedi KL. “Improved bioavailability of dapsone in the presence of piperine in rats,” Pharmaceutical Sciences 1997; 03:189-191.
12. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998; 64(4):353-356.
13. Singh NK, Kumar P, Gupta DK, Singh S, Singh VK. “UV spectrophotometric method development for estimation of piperine in Chitrakadi Vati”. Der Pharmacia Letter 2011; 3:178-82.
14. Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, et al. Piperine inhibits TNF-alpha induced adhesion of neutrophils to endothelial monolayer through suppression of NF-kappaB and IkappaB kinase activation. Eur J Pharmacol. 2007; 575(1-3):177-186.
15. Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010; 122(3):777-785.
16. Makhov P, Golovine K, Canter D, Kutikov A, Simhan J, et al. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate. 2012; 72(6):661-667.
17. Stöhr JR, Xiao PG, Bauer R. Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol. 2001; 75(2-3):133-139.
18. Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett. 2011; 203(1):9-19.
19. Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012; 33(4):523-530.
20. Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010; 122(3):777-785.
21. Makhov P, Golovine K, Canter D, Kutikov A, Simhan J, et al. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate. 2012; 72(6):661-667.
22. Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, et al. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004; 279(24):25535-25543.
23. Eom YW, Kim MA, Park SS, Goo MJ, Kwon HJ, et al. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene 2005; 24(30):4765-4777.
24. Yokochi T, Robertson KD. Doxorubicin inhibits DNMT1, resulting in conditional apoptosis. Mol Pharmacol. 2004; 66(6):1415-1420.
25. Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis. 2002; 19(8):703-708.
26. Horwitz SB, Lothstein L, Manfredi JJ, Mellado W, Parness J, et al. “Taxol: mechanisms of action and resistance”. Ann. NY Acad Sci. 1986: 466:733–744.
27. Saunders DE, Lawrence DW, Christensen C, Wappler NL, Ruan H, et al. “Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells”. Int J Cancer 1997; 70:214–220.
28. El-Kareh AW, Secomb TW. Two-mechanism peak concentration model for cellular pharmacodynamics of Doxorubicin. Neoplasia. 2005; 7(7):705-713.
29. Blagosklonny MV, Schulte TW, Nguyen P, Mimnaugh EG, Trepel J, et al. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer Res. 1995; 55(20):4623-4626.
30. Yu D, Jing T, Liu B, Yao J, Tan M, et al. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998; 2(5):581-591.