Abstract
Background: Pineal parenchymal tumors (PPTs) are uncommon tumors comprising of pineocytoma (PC), pineal parenchymal tumor of intermediate differentiation (PPTID) and pineoblastoma (PB). Morphological sub typing and histological grading based on mitotic index and neurofilament (NF) immunostaining are the factors affecting the survival of these patients. Treatment strategy and prognosis of PPTIDs remain controversial with limited data available on pathologic features and biologic behavior of PPTID.
Case series: We report a series of 8 pineal parenchymal tumors over a period of 5 years with special reference to PPTIDs. The series includes 3 cases of PC, 2 of PPTID and 3 of PB. Patients underwent decompression, microsurgical/ stereotactic/ endoscopic biopsy. Histological features with MIB1 LI (labelling index) and NF immunostaining were studied and showed varied presentation. One case each of PC and PPTID showed ganglion like cells. Both PPTIDs showed 8% and 20% MIB1 LI. All PBs showed brisk mitosis hemorrhage and necrosis except for one case where mitosis was not clearly evident but showed high MIB1 LI (50%).
Conclusion: PCs with ganglionic differentiation have an essentially benign course.
Ganglionic differentiation in PPTIDs, its impact on the prognosis and as a differentiating factor between PPTID grade II and grade III needs further study.
Keywords: Pineocytoma; pineal parenchyma; pineoblastoma; MIB1 LI; neurofilament; synaptophysin
Full Text
The World Health Organization (WHO) classification scheme, released in 2007, categorizes pineal parenchymal tumors into 3 subtypes and different grade categories: 1) WHO grade I pineocytomas (PC), 2) WHO grade II or III pineal parenchymal tumors of intermediate differentiation (PPTID), and 3) WHO grade IV pineoblastomas (PB). Pineocytomas are the lowest grade tumors. Pineal parenchymal tumors of intermediate differentiation (PPTID) share some features with both pineocytomas and pineoblastomas, but generally lack the more definitive diagnostic features that define these two extremes. Pineoblastomas are highly malignant tumors. We describe a series of 8 pineal parenchymal tumors at our center reported over a period of 5 years with special reference to PPTIDs. The case series includes 3 cases of PC, 2 cases of PPTID and 3 cases of PB. Patients underwent microsurgical biopsy/ decompression, stereotactic or endoscopic biopsy after which squash smears were prepared for few cases. NF, synaptophysin and GFAP immunostaining, MIB1 labelling index (LI) were done wherever possible after study of histological features. PPTIDs were graded based on mitotic index and NF staining [7].
Case presentation
Pineocytomas
Case 1-3
The three cases of PC (Table 1) presented with headache, vomiting and concomitant hydrocephalus. One case had urinary incontinence and cognitive problems. On histology the tumors were composed of sheets of round cells arranged in pineocytomatous rosettes with short processes and round finely dispersed chromatin and inconspicuous nucleoli (Figure 1a). All the cases showed MIB1 LI up to 4%. One case was characterized by presence of large pleomorphic often multinucleated cells (Figure 1b) along with isomorphic round cells. The pleomorphic cells showed NF positivity (Figure 1c).
Table 1: Pineocytoma cases, neurofilament (NF) staining.
|
Case
|
Age (years) /Sex
|
Mitosis (per10HPF)
|
NF staining
|
Mib LI
|
Followup
|
Other
findings
|
|
Case 1
|
52/M
|
1
|
Positive
|
4%
|
No follow-up
|
-
|
|
Case 2
|
38 /F
|
1
|
Positive
|
4%
|
4 years without recurrence
|
-
|
|
Case 3
|
65/M
|
1
|
Positive
|
4%
|
No follow-up
|
Ganglionic
differentiation
|
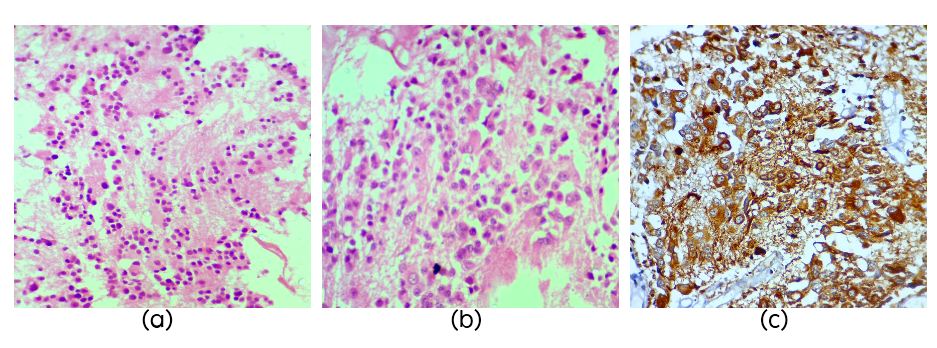
Figure 1: Section showing pineocytomatous rosettes (Figure 1a, H&E, x40), section showing ganglion like cells in pineocytoma (Figure 1b, H&E, x40) and NF staining which is strongly positive in ganglion cells in pineocytoma (Figure 1c).
Pineal parenchymal tumors of intermediate differentiation
Case 4
A 29-years-old male was admitted complaining of headache and vomiting, MRI showed a mass in posterior third ventricle in pineal region. The tumor was totally excised through right combined supra and infra tentorial approach which upon microscopy demonstrated a tumor composed mostly of small cells in sheets and ill-defined lobules and foci of rosette formation. Focal areas showed moderate pleomorphism, multinucleate and ganglion like cells with low mitotic activity (1-2/10hpf). MIB1 LI was 20% while synaptophysin was positive (Table 2).
Table 2: Cases of pineal parenchymal tumor of intermediate differentiation.
|
Case
|
Age (years) /Sex
|
Mitosis (per10HPF)
|
NF staining
|
Mib LI
|
Follow-up
|
Other
findings
|
|
Case 4
|
27/M
|
2
|
Positive
|
20%
|
5 years without recurrence
|
Ganglionic
differentiation
|
|
Case 5
|
60/ F
|
1
|
Positive
|
8%
|
Recurred after 5 years
|
-
|
Case 5
A 60-years-old female with a history of pineal tumor with hydrocephalus underwent endoscopic third ventriculostomy in 2010 and later presented with mass in the pineal region. Per operatively the tumor was grey white soft moderately vascular. Right occipital and suboccipital craniotomy with supra and infra tentorial approach was performed and a near total excision of the tumor was done. The tumor was composed of cells arranged in sheets (Figure 2a) and vague lobules separated by capillaries. There were multiple pineal rosettes and all the cells showed strong NF staining (Figure 2b) and mitotic activity was sparse (1/10Hpf) with MIB1 LI of 8% (Figure 2c).
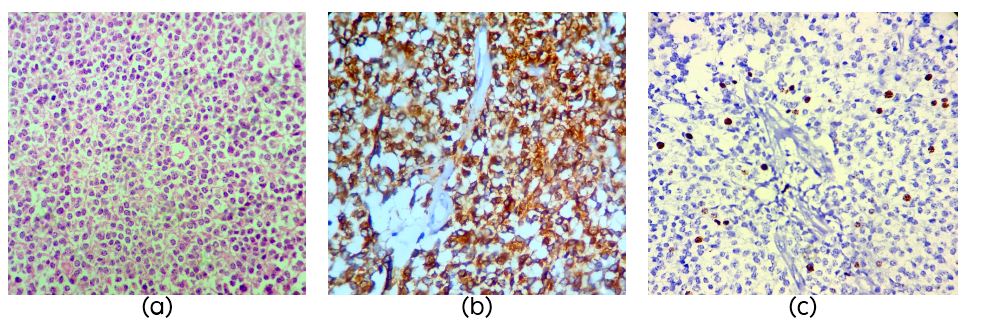
Figure 2: Section showing round mild pleomorphic cells in sheets in a case of pineal parenchymal tumor of intermediate differentiation (PPTID) (Figure 1a, H&E, x40); Strong NF staining in PPTID (Figure 1b, H&E, x40) and MIB1 LI of 8% in PPTID (Figure 1c).
Pineoblastomas
Cases 6-8
PB was diagnosed in 3 patients aged 4, 17 and 41years (Table 3). Two cases had recurrence, one after 8 years and the other after 3 years. On histology the tumors were highly cellular composed of small cells (Figure 3a) with high NC ratio, round to oval hyperchromatic nuclei with granular irregularly dispersed chromatin and occasional small nucleolus. NF was positive in one case (Figure 3b). Mitotic activity was brisk in two cases but not clearly evident in one case which showed MIB1 LI of 50% (Figure 3c). Necrosis was seen in two cases.
Table 3: Pineoblastoma cases.
|
Case
|
Age (years) /Sex
|
Mitosis (per10HPF)
|
NF staining
|
MIb LI
|
Follow-up
|
Other
findings
|
|
Case 6
|
4/M
|
18
|
NA
|
NA
|
Recurrence after 3 years
|
Necrosis
|
|
Case 7
|
17/F
|
17
|
NA
|
NA
|
2 years without recurrece
|
Necrosis
|
|
Case 8
|
41/M
|
Not clear
|
Positive
|
50%
|
Recurrence after 8 years
|
Apoptotic
bodies
|
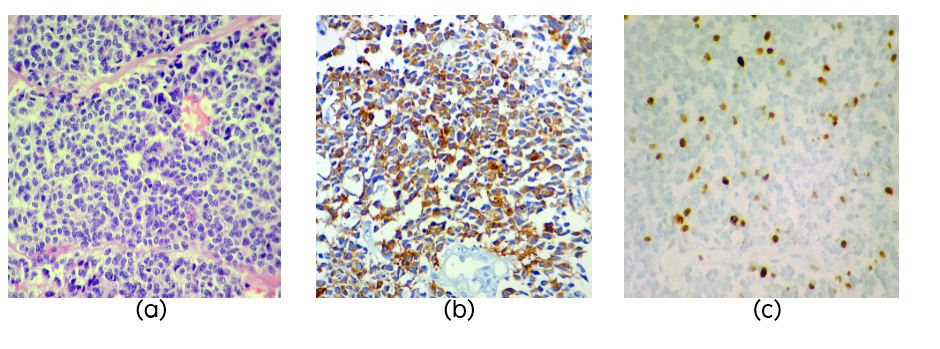
Figure 3: Section showing pleomorphic cells in sheets in a case of pineoblastoma (PB) (Figure 1a, H&E, x40), NF staining in PB (Figure 1b) and high MIB1 LI in PB (Figure 1c).
Discussion
PCs show uniform cells arranged in a diffuse or loosely lobular growth pattern. The nuclei are round, oval, or sometimes indented, with a thin or “salt and pepper” chromatin along with pineocytomatous rosettes [2]. Some cases of PC also show pleomorphic cells which indicate ganglionic or astrocytic differentiation. Early studies have suggested a more benign clinical course for pineocytomas with neuronal or neuronal and astrocytic differentiation [3].
PPTIDs share some features with both PCs and PBs. Morphologically, PPTIDs exist in 3 separate subtypes, including 1) the endocrine-like subtype with lobulated growth pattern and increased vascularity, 2) the oligodendroglioma/neurocytoma-like type with diffuse growth patterns, and 3) transitional type with areas of lobulated and/or diffuse growth patterns intermixed with focal pineocytoma-like regions containing well-formed pineoctyomatous rosettes [4]. Jouvet and colleagues, proposed a new system dividing PPTIDs into two subgroups based on their histology. Low-grade PPTIDs, representing WHO grade II, can have any of the 3 growth patterns described (transitional, lobulated, or diffuse), and have high expression of neurofilament, similar to pineocytomas. The low-grade PPTIDs also have 0 to 5 mitoses per 10 HPF, with moderate MIB1 indices. High-grade PPTIDs are WHO grade III, do not contain any pineocytoma-like regions, and hence have lobulated or diffuse growth patterns with very limited neurofilament expression, reflecting a more limited degree of neuronal differentiation in comparison with lower-grade examples. The mitotic index is also higher, with typically more than 5 mitoses per 10 HPF encountered, and high MIB-1 labeling indices. Vascular proliferation and necrosis are also more commonly found in high-grade PPTIDs [5].
PPTIDs showed <5 mitosis, strong NF positivity with significantly high MiB LI and recurrence was seen in one case with no cerebrospinal fluid (CSF) seeding or metastasis. Both the PPTIDs showed significantly high MIB1 LI compared to previous studies [6]. A case of PPTID showed ganglionic differentiation with MIB1 LI of 20% which is seen rarely and also may have a favorable clinical course [7].
PBs may have ill-defined borders and grow invasively into surrounding tissue. The tumor shows marked hypercellularity with variable growth patterns, along with variable degrees of necrosis. Individual tumor cells contain minimal cytoplasm with high nuclear-cytoplasmic ratios and frequent mitotic figures. Focal expression of neuronal markers is usually present, and there is also variable focal positivity for glial fibrillary acidic protein. The mitotic activity and necrosis in one of our cases was not clearly evident but MIB1 LI was 50%.
Conclusion
PCs with ganglionic differentiation have an essentially benign course. Ganglionic differentiation in PPTIDs, its impact on the prognosis and as a differentiating factor between PPTID grade II and grade III needs further study. MIB1 LI would be helpful in samples where mitotic activity is not clearly evident and its usefulness in grading PPTIDs needs to be clearly defined.
Acknowledgements
We are thankful to Departments of Pathology, and Neurosurgery, KIMS hospitals for their support.
Conflicts of interest
There are no conflicts of interest.
References
1. Nakazato AY. Jouvet BW. Scheithauer: WHO classification of tumors of the central nervous system. Lyon (France): IARC Press, 2007.
2. Dahiya S, Perry A. Pineal tumors. Adv Anat Pathol. 2010; 17(6):419–427.
3. Herrick MK, Rubinstein LJ. The cytological differentiating potential of pineal parenchymal neoplasms (true pinealomas). A clinicopathological study of 28 tumours. Brain. 1979; 102(2):289–320.
4. Fauchon F, Jouvet A, Paquis P, Saint-Pierre G, Mottolese C, et al. Parenchymal pineal tumors: a clinicopathological study of 76 cases. Int J Radiat Oncol Biol Phys. 2000; 46(4):959–968.
5. Jouvet A, Saint-Pierre G, Fauchon F, Privat K, Bouffet E, et al. Pineal parenchymal tumors: a correlation of histological features with prognosis in 66 cases. Brain Pathol. 2000; 10(1):49–60.
6. Fèvre-Montange M, Vasiljevic A, Frappaz D, Champier J, Szathmari A, et al: Utility of Ki67 immunostaining in the grading of pineal parenchymal tumours: a multicentre study. Neuropathol Appl Neurobiol. 2012; 38(1):87–94.
7. Fèvre-Montange M, Szathmari A, Champier J, Mokhtari K, Chrétien F, et al. Pineocytoma and pineal parenchymal tumors of intermediate differentiation presenting cytologic pleomorphism: a multicenter study. Brain Pathol. 2008; 18(3):354–359.