Case Report
2017
September
Volume : 5
Issue : 3
Primary melanocytic tumor of the cerebellopontine angle mimicking acoustic schwannoma: Case report
Raghavendra H, Dilip Kumar, Vikrant Kesri, Manas Kumar Panigraghi, Sailaja Reddy M
Pdf Page Numbers :- 106-110
Raghavendra H1,2,*, Dilip Kumar1, Vikrant Kesri1, Manas Kumar Panigraghi1 and Sailaja Reddy M3
1Department of Neurosurgery, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
2Department of Neurosurgery, ESIC Medical College and Super Speciality Hospital, Hyderabad-500038, Telangana, India
3Department of Pathology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
*Corresponding author: Dr. Raghavendra H, Department of Neurosurgery, ESIC Medical College and Super Speciality Hospital, Hyderabad-500038, Telangana, India. Email: harpanahalli.raghavendra@gmail.com
Received 23 March 2017; Revised 25 May 2017; Accepted 9 June 2017; Published 19 June 2017
Citation: Raghavendra H, Kumar D, Kesri V, Panigraghi MK, Sailaja M. Primary melanocytic tumor of the cerebellopontine angle mimicking acoustic schwannoma: Case report. J Med Sci Res. 2017; 5(3):106-110. DOI: http://dx.doi.org/10.17727/JMSR.2017/5-20
Copyright: © 2017 Raghavendra H et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Intra-cranial melanomas are commonly metastatic, from primary melanoma elsewhere in the body. The primary occurrence of a melanoma in the brain parenchyma is rare. We report a case of 26-year aged woman who presented with headache, hearing loss, mild cerebellar signs. On imaging, a space occupying lesion was found in the right cerebellopontine angle and a preoperative diagnosis of schwannoma/ meningioma was made. She underwent right retro-mastoid sub-occipital craniectomy and excision of a blackish vascular tumour. Histopathological examination revealed a melanocytoma intermediate grade, mib Index 4-5%. Search for dermal, mucous and ocular lesions were negative. She received image guidance adjuvant radiation to the post- operative tumour bed to 50.4 Gy/28 fractions @ 180 cGy tumor. She tolerated the treatment well and is symptom free 12 months after treatment. We report a rare case of primary melanocytic tumour of the cerebellopontine angle.
Keywords: cerebellopontine angle; primary melanoma
Full Text
Case report
A 25-year-old female presented with headache, diminished hearing on right ear, difficulty in walking. On examination, her higher mental functions were normal with right ear sensoneural hearing loss with mild cerebellar signs. Magnetic resonance imaging (MRI) revealed a hypointense lesion in the right cerebellopontine cistern on T2-weighted images that was hyperintense on T1-weighted images (Figures 1). The lesion showed enhancement after contrast infusion, with adjacent satellite lesion (Figure 1). Magnetic resonance spectroscopy (MRS) demonstrated minimal increase in the choline/NAA ratio. The possibility of an acoustic schwannoma and meningioma was kept as differential diagnosis. After pre-operative work up, the patient was scheduled for right suboccipital craniectomy. Blackish discoloration of both inner and outer surface of dura was noted with pigmented spots on cerebellar surface (Figure 2). Extra axial blackish firm to tough, vascular tumor mass not easily amenable with CUSA with pigments attach to V to XI cranial nerve and anterior inferior cerebellar artery (AICA), superior petrsol vein and brain stem (Figure 3). Microscopic examination revealed a cellular neoplasia composed of large cells with vesicular nuclei and prominent nucleoli. Several cells showed dense intra-cytoplasmic deposits of melanin pigment (Figure 4a), IHC reveals diffuse positivity for HMB-45 (Figure 4b) & S100b (Figure 4c) with Mib index of 4-5 % (Figure 4d). Thus a diagnosis of melanocytoma intermediate grade was made. A thorough search of the primary consisting of dermatological evaluation, ophthalmological evaluation, X-ray of the chest and ultrasonography of abdomen was fruitless. We therefore concluded the lesion to be primary melanoma of the cerebellopontine angle. The immediate postoperative stage was uneventful. She received image giuidance adjuvant radiation to the post- operative tumour bed to 50.4 Gy/28 fractions @ 180 cGy. She tolerated the treatment well and is symptom free for twelve months after treatment.
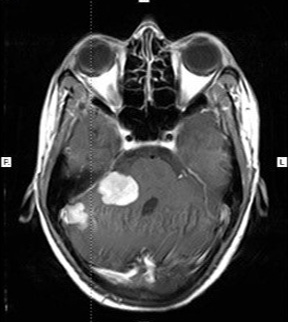
Figure 1: MRI brain.
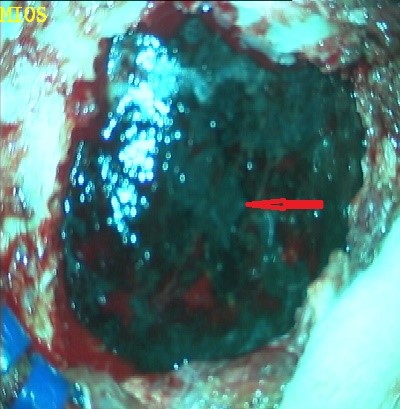
Figure 2: Dural pigmentation.
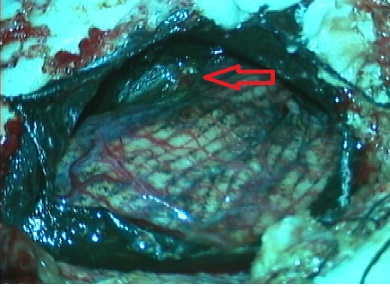
Figure 3: Tumor pigmentation with cerebellar surface.
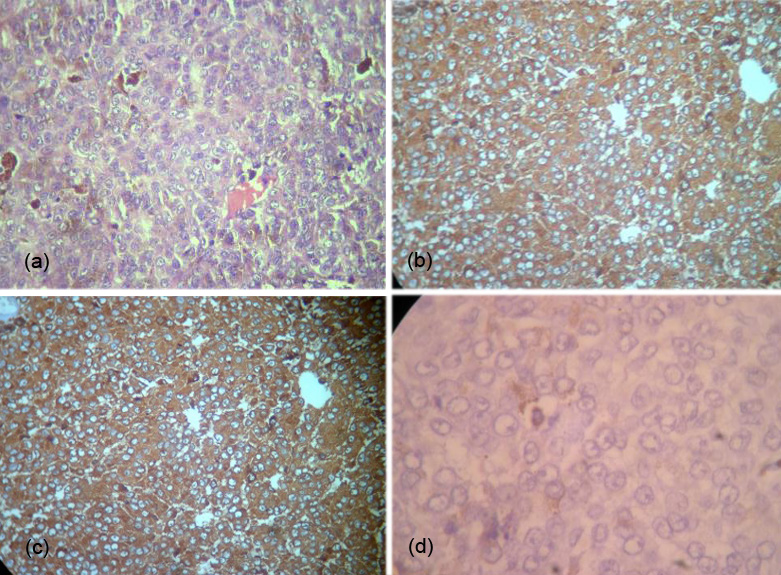
Figure 4: (a) Melonocytic granules, (b) IHC positive for HMB 45, (c) IHC positive for S100B, (d) IHC positive for MIB 4-5%.
Discussion
Leptomeningeal melanocytes are derived from the neural crest during early embryonic development. Melanocytic melanin is similar to skin melanin and different from neuromelanin, which is found exclusively in neurons. The largest concentration of melanocytes is on the piamater surrounding the medulla and high cervical cord [5], and this may reflect the common location of melanocytomas. Some authors have proposed that dural melanomas may have a possible origin from leptomeningeal deposits where the leptomeninges penetrate into the dura [6]. Alternately, heterotopic tissues have been described in dura mater and it is possible for a melanoma to arise from a heterotopic mass of melanocyte producing neural crest cells [7].
Pathology
Depending on the biological behavior, melanocytomas are classified into [8]:
Well-differentiated melanocytoma: These are the benign type, containing variably pigmented melanocytic cells arranged in tight nests, sheets, or fascicles. Nuclei are regular, often with small, eosinophilic nucleoli. Mitotic rates range from zero to one per 10 high-power fields (HPF) and MIB-1 staining is low (<1-2%). They tend to occur in the spinal canal and are unlikely to recur after excision.
Malignant melanoma: These lie at the other end of spectrum and are the most malignant type. They consist of larger, cytologically atypical, pigmented tumor cells with bizarre, pleomorphic nuclei growing in loose nests or sheets, often with CNS invasion or necrosis. They have high mitotic rates (about 5.7/10 HPF) and high MIB-1 labeling index (about 8.1%). They have a high propensity to recur and a poor prognosis.
Intermediate grade melanocytoma: The intermediate grade melanomas are difficult to define. The histopathological features of intermediate-grade melanocytic tumors lie in between that of melanocytoma and melanoma. They have sheet-like growth patterns, microscopic CNS invasion, and occasional mitoses. MIB-1 staining ranges from 1% to 4%. It is therefore likely that these three lesions are the spectrum of a same disease rather than three different clinical entities.
All three types are immunoreactive for, HMB-45 and S-100 protein and negative, for epithelial membrane antigen [2].
Clinical presentation
Melanomas of the CNS have a peak at the 4th- 5th decades and have a male preponderance. Like all melanomas they are less common in blacks [2]. Gibson in 1957 classified primary leptomeningeal melanoma into two groups: Group A or diffuse variety is the rarer one, and more common in adults than in children [7]. In children they are associated with neurocutaneous melanosis [6]. Clinically they present with features of raised intracranial pressure and are difficult to pick up on CT/MRI. The antemortem diagnosis is notoriously difficult and it mimics a variety of conditions like lymphoma, leukemia, metastatic carcinoma, subacute meningitis, viral encephalitis, and idiopathic hypertrophic cranial pachymeningitis [9]. Group B or the discrete variety is the commoner of the two, and presents with features of raised intracranial tension with focal signs and meningism [2]. These cases are easily picked up on CAT/MRI. While they are usually diagnosed as an intracranial space occupying lesion, the exact pathology and malignant nature are difficult to assess preoperatively. They have a relatively better prognosis.
Diagnosis
The typical CAT finding is a contrast enhancing hyperdense mass. On MRI, the classical finding is of a hyperintense lesion on T1 and hypointense lesion on T2. There have been few cases reported with hypointense T1 and iso-high intese T2 WI. These variations are thought to be due to the degree of paramagnetic effect of stable free radicals in melanin and intratumoral haemorrhage [10]. Although CAT/MRI point towards the presence of melanin, the exact pathology cannot be determined. It is also difficult to determine whether the lesion is benign or malignant. The only clue on imaging is leptomeningeal dissemination, which is difficult to pick up on CAT/ MRI. There is not enough data to suggest any advantage of MRS. MRS in our case demonstrated only a minimal increase in choline/ NAA ratio and pointed towards a benign disease. PET CT plays a significant role in the diagnosis of primary CNS malignant melanoma. The final diagnosis is made by the pathologist, based on the mitotic figures, nuclear atypia and MIB 1 index. If the lesion is at the CP angle, like in our case, it must be differentiated from melanotic schwannoma. Melanotic schwannomas are well circumscribed, partly encapsulated lesions characterized by polygonal and vesicular cells with grooved nuclei. These cells also contain abundant melanin pigment. Immuno-histochemical markers used are S100 (a protein expressed in cells of neural crest origin), HMB 45 (detects melanosomes) and Melan. Among these S100 has high sensitivity, while HMB 45 has high specificity. Since melanocytomas of all grades as well as melanin containing tumours like melanotic schwannomas react positively, they help little to differentiate between these pathological types [10, 9].
Management
Surgical excision is the primary mode of treatment. Total excision is better than subtotal excision, not only for melanomas but also in case of melanotic schwannoma and intermediate grade melanoma [11, 12, 13]. Total excision anyhow would be impeded by involvement of lower cranial nerves and major vessels. Use of adjuvant therapies do not share such a similar consensus. Traditionally chemotherapy and radiotherapy (RT) had no role in primary CNS melanomas, while some chemotherapeutic agents like temozolamide and dacarbazine were used in metastatic melanoma [14]. RT using fractionized irradiation in high doses has improved response rates for melanomas [15]. Gamma knife radiotherapy has shown some benefit in intermediate grade melanocytomas [12]. Among chemotherapeutic agents, DTIC when used along with1-(4-amino-2methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride (ACNU), and vincristine with OK 432 (picibanil) has shown promising results [15].
Prognosis
Melanomas are notorious for their dismal prognosis. The factors determining prognosis are, type of lesion, involvement of leptomeninges, Amount of tumor excised and MIB 1 index. Among these the most significant one is MIB 1 index.
Conclusion
Primary CNS melanoma is difficult to diagnose preoperatively. Typically the MRI picture, gross features of the tumor, MIB-1 proliferative index, immunohistochemistry and 18F-FDG PET CT play major role in the diagnosis of primary CNS malignant melanoma. Radical excision is the main modality of treatment. Concurrent chemoradiation in adjuvant setting for primary CNS malignant melanoma is beneficial.
Conflicts of interest
Author declares no conflicts of interest.
References
[1] Schuchter LM, Haluska F, Fraker D, Elenitsas R. Skin: Malignant melanoma In: Abeloff MD (ed) Clinical Oncology. New York: Churchill Livingstone, 2000:1326–1327.
[2] Rodriguez y Baena R, Gaetani P, Danova M, Bosi F, Zappoli F. Primary solitary intracranial melanoma: Case report and review of literature. Surg Neurol. 1992; 38(1):26–37.
[3] Whinney D, Kitchen N, Revesz T, Brookes G. Primary malignant melanoma of the cerebellopontine angle. Otol Neurotol. 2000; 22(2):218–222.
[4] Liu XW, Chi ZF, Zhao XE, Wu W. The clinical features and meningeal histochemistry of meningeal malignant melanosis. Chin Med J. 2008; 121(23):2458–2460.
[5] Arbelaez A, Castillo M, Armao MD. Imaging features of intraventricular melanoma. AJNR Am J Neuroradiol. 1999; 20(4):691–693.
[6] Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: Definition and review of the literature. Acad Dermatol. 1991; 24 (5 Pt 1):747–755.
[7] Narayan RK, Rosner MJ, Povlishock JT, Girevendulis A, Becker DP. Primary Durla Melanoma: A clinical and morphological study. Neurosurgery. 1981; 9(6):710–716.
[8] Laxminarayan B, Jacob A, Aparna G, Sreekumar T. Primary cerebellopontine angle melanoma: A case report and review. Turkish Neurosurgery. 2012; 22(4):469–474.
[9] Pirini MG, Mascalchi M, Salvi F, Tassinari CA, Zanella L, et al. Primary diffuse meningeal melanomatosis: Radiologic-pathologic correlation. AJNR Am J Neuroradiol. 2003; 24(1):115–118.
[10] Kashiwagi N, Hirabuki N, Morino H, Taki T, Yoshida W, et al. Primary solitary intracranial melanoma in sylvian fissure: MR demonstration. Eur Radiol. 2002; 12 Suppl 3:S7–10.
[11] Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasm of the central nervous system. Am J Surg Pathol. 1999; 23(7):745–754.
[12] Hamasaki O, Nakahara T, Sakamoto S, Kutsuna M, Sakoda K. Intracranial meningeal melanocytoma. Neurol Med Chir (Tokyo). 2002; 42(11):504–509.
[13] Piedra MP, Scheithauer BW, Driscoll CL, Link MJ. Primary Melanocytic tumor of the cerebellopontine angle mimicking a vestibular schwannoma: Case report. Neurosurgery. 2006; 59(1):E206.
[14] Khan P, Shelton C, Townsend J, Jensen R. Primary malignant cerebellopontine angle melanoma presenting as a presumed meningioma: Case report and review of the literature. Skull base. 2003; 13(3):159–166.
[15] Oh JY, Joo W, Rha HK, Kim YW. Primary occipital malinant melanoma. J Korean Neurosurg Soc. 2007; 41:39–42.