Full Text
Introduction
The burden of cervical cancer in India is enormous, accounting for about 20 per cent of all cancer related deaths in women and it is the number one cause of death in middle aged Indian women [1]. Cervical cancer is one of the most common cancers among women worldwide (WHO, 2009b). Its mortality exemplifies health inequity, as its rates are higher in low & middle income countries (LMICs) (WHO, 2009b), and in low socio-economic groups within the countries [2]. Around 80% of global cervical cancer cases are in LMICs [3] (WHO, 2009a). Cervical cancer is a deadly disease once it reaches the invasive stages, but out of all the female genital tract cancers, it is the only preventable cancer if detected at its early stages.
Cervical cancer is often linked with sexually transmitted infections and extramarital relationships. These are not culturally accepted in India, as a result, women refused to be screened out of fear of the potential social stigma. Studies conducted in different setting in India suggest that women were aware of risk factors like sexual activity at an early age, extramarital sexual relationships associated with cervical cancer [4]. In addition, women were often opposed by men from accessing cervical cancer screening services [5]. Cultural factors also affect wives ability to make decision since husbands have to be consulted or informed before seeking any treatment [6].
Cancer incidence data in India
Cancer incidence data collected by the registries of Bangalore, Bhopal, Chennai, Delhi, Mumbai, Sikkim, Manipur, Mizoram, Dibrugarh and Aizawl for the year 2006-2008 and respective population estimates estimated earlier along with the population figures reported by the Census 2011, formed the sources of data for the present analysis. In general, age adjusted rates (AARs) did not differ significantly by the two approaches for all the five selected leading sites and for all the registries. The comparison of AARs by two approaches for the leading sites of breast, cervix, ovary, lung and oesophagus for females during 2006-2008 is shown in Table 1. As in the case of males, in general, the AARs did not differ significantly by two approaches for all the five selected leading sites and for all the registries [7].
Table 1: Comparison of age adjusted rates (2006-2008) by different methods in selected registries-Females [7].
|
|
Breast
|
Cervix
|
Ovary
|
Lung
|
Oesophagus
|
|
|
A
|
B
|
A
|
B
|
A
|
B
|
A
|
B
|
A
|
B
|
|
Bangalore
|
36.1
|
36.0
|
21.1
|
21.1
|
7.8
|
7.8
|
3.9
|
3.8
|
6.4
|
6.4
|
|
Bhopal
|
25.4
|
26.6
|
18.9
|
19.9
|
7.7
|
8.1
|
2.7
|
2.8
|
5.4
|
5.7
|
|
Chennai
|
31.5
|
32.4
|
18.5
|
19.1
|
7.4
|
7.6
|
4.1
|
4.2
|
4.3
|
4.4
|
|
Delhi
|
32.3
|
36.2
|
17.9
|
20.0
|
8.9
|
10.0
|
3.6
|
4.0
|
2.8
|
3.1
|
|
Mumbai
|
32.3
|
34.9
|
14.0
|
15.2
|
7.1
|
7.7
|
3.8
|
4.1
|
2.9
|
3.2
|
|
Sikkim
|
7.2
|
7.9
|
10.9
|
12.0
|
4.2
|
4.7
|
7.7
|
8.5
|
7.4
|
8.1
|
|
Manipur
|
8.4
|
8.2
|
9.4
|
9.2
|
4.0
|
3.9
|
12.4
|
12.1
|
1.9
|
1.9
|
|
Mizoram
|
15.1
|
15.5
|
17.6
|
18.0
|
3.0
|
3.1
|
26.3
|
26.9
|
3.0
|
3.1
|
|
Aizawl Dist
|
23.3
|
23.5
|
22.4
|
22.6
|
4.1
|
4.1
|
38.7
|
39.0
|
4.9
|
4.9
|
|
Dibrugarh
|
12.1
|
12.2
|
6.1
|
6.2
|
5.6
|
5.6
|
1.7
|
1.7
|
11.4
|
11.6
|
Abbreviations: A: Rates Arrived Using the population estimates based on 1991-2001; B: Rates arrived using the population estimates based on 2001-2011.
Cervical cancer
In early 1980’s Prof. Zurhausen and his collegues identified the association between HPV infection and cancer cervix in 99.7% cases. HPV types 16, & 18 are responsible for more than 75% of cervical cancers and more than 50% of vaginal and vulvar cancers. HPV infection may clear in 1 year or it may procede to develop into full blown carcinoma (Figure 1). The average time for complete carcinogenesis is around 10 to 12 years. Cervical mucosa goes through a series of changes before developing into full blown carcinoma. HPV infection: 0-1yr, lowgrade lesions: 0-5yrs, highgrade lesions: 0-20yrs, Invasive cancer.
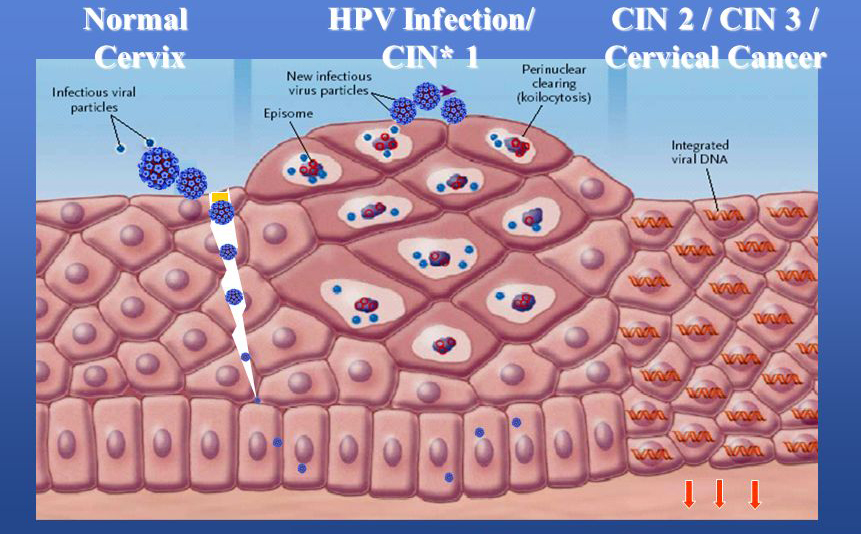
Abbreviations: CIN*: cervical intraepithelial neoplasia
Figure 1: Spectrum of changes in cervical squamous epithelium caused by HPV infection [8].
Premalignant intraepithelial dysplasia (Low-grade squamous intraepithelial lesions(LSIL)), High-grade squamous intraepithelial lesions (HSIL) or cervical intraepithelial neoplasia grades 1-3 (CIN1-3) is generally asymptomatic but local symptoms such as, pruritus, irritation, dyspareunia, and abnormal vaginal discharge may occur (Figure 2).
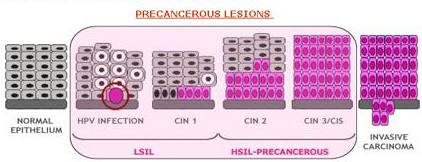
Figure 2: The major steps in cervical carcinogenesis, before infection of the metaplastic epithelium of the cervical transformation zone with one or more carcinogenic HPV types, viral persistence rather than clearance, donal progression of the persistently-infected epithelium to precancerous lesions, and invasion.
Prevention
Since this cancer is a slow growing one, it gives many opportunities to detect it early & 100% preventable if diagnosed early. Primary prevention: a) Health promotion & education, b) Vaccination; Secondary prevention: a) Screening, b) Early diagnosis & treatment; Tertiary prevention: a) Treatment, b) Palliation & rehabilitation.
Health promotion & education: Acording to WHO health education and promotion should be an integral part of any National Cervical Cancer Control programme. It should incorporate an awareness component, aspects of behaviour modification and counselling.
Vaccination: Vaccination may provide a solution to prevention. Different vaccines have been developed, Gardasil (Merck) against 16,18,6 and 11 another one is Cervarix (GSK) against types 16 and 18. Both vaccines need to be administered in 3 doses over a 6 month period between 9 to 26 Years, and are most effective if given before 1st sexual encounter.
Screening methods available
1) Cytology: Pap smear (1949) & LBC (Liquid based cytology 1996); 2) HPV DNA test: High risk HPV DNA test/ HPV typing (2000’s); 3) Testing in resource poor areas: Visual inspection of cervix after application of 3-5% acetic acid (VIA) or Lugol’s iodine(VILI); 4) Follow up with colposcopy or directed biopsy or treat with ablative methods or excisional methods.
Pap smear: Optimum time for this test is 10th to 16th day of cycle. Lubricants and Bi-manual examination should be avoided (Figures 3A, B, C, D, E, F, G, H)
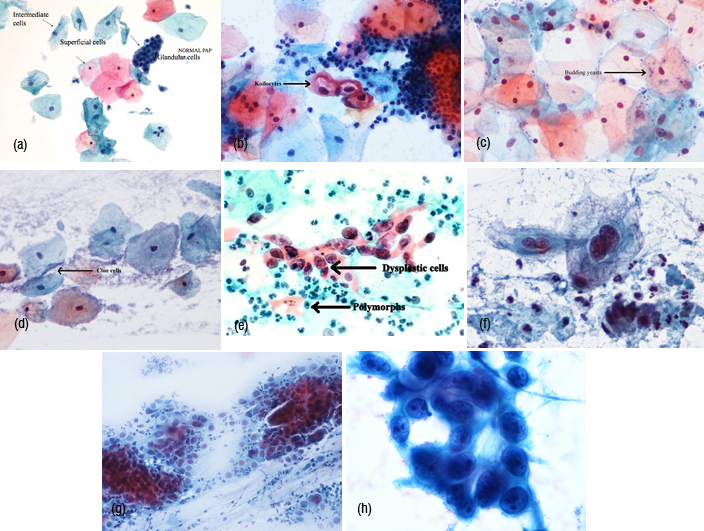
Figure 3: Pap smear (A) Normal smear, (B) Koilocytes, (C) Budding yeast, (D) Clue cells, (E) Dysplastic cells, (F) Lowgrade SIL, (G) High grade SIL, (H) Adeno carcinoma.
Liquid base cytology: This test is introduced in 2003 in western countries. The principle behind LBC is that instead of smearing the spatula across slide, it is rinsed in a vial of preservative solution. In the Lab. the vial is agitated, so that there is uniform distribution of cells which are then filtered out under pressure to produce a thin layer of cells on a slide. It also allows the possibility of other tests such as HPV testing from the same sample. The Comparison of Pap and LBC are included in the Table 2.
Table 2: Comparison of PAP and LBC.
|
PAP smear
|
Liquid base cytology
|
|
Cell presentation - pictured is a portion of one field of view as a cyto-technologist might see it looking through a microscope (Figure 4).
|
Both views contain abnormal cell nuclei. However, the abnormal cells on the right are considerably easier to see (Figure 5).
|
|
Heterogeneous
Graphic cell localization
300-500 cells/slide
Variable fixation
Thick uneven groups
need frequent focusing
Dirty background
Variable preservation
False Negative rates higher than 50%.
Compromised by the presence of blood, mucous, obscuring inflammation, scant cellular material and air-drying artefact. Furthermore, the three-dimensional (3D) character of overlapping cells in lumps and clumps makes the examination a daunting challenge.
|
Homogeneous
Random cell presentation
50-70 cells/slide
Uniform fixation
Uniform thin layer
Not single cell, mono layers
Clean background
Well preserved cells
False Positive and False Negative (error rates) were cut by a factor of 4
Increases disease detection 65%.
This method preserves the cells and minimizes cell overlap, blood, mucus, and inflammation. It creates a mono-layer, a layer one cell thick, with no overlapping cells.
|
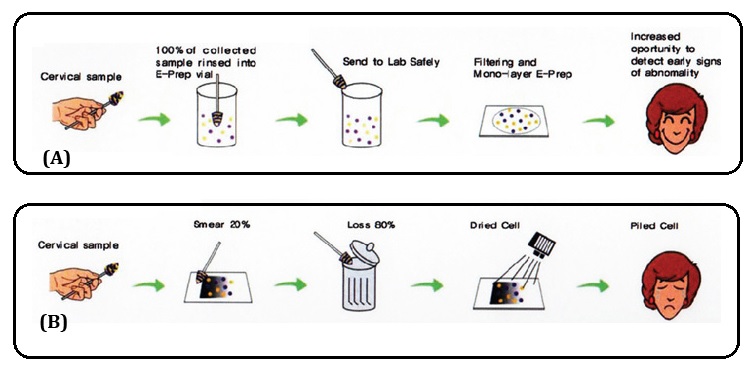
Figure 4: (A) Liquid-based Pap test (E-Pap), (B) Pap smear
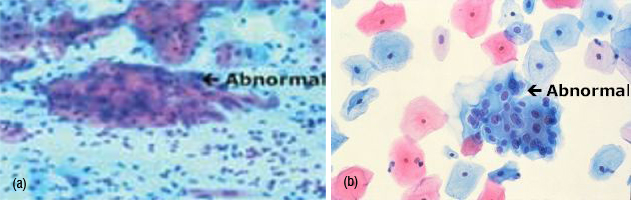
Figure 5: Liquid base cytology.
HPV DNA testing: Molecular tests can detect DNA from cancer causing HPV types in vaginal or cervical smears collected using a small brush or swab, either by trained providers or by women by themselves. This test is particularly valuable in detecting high grade precancerous lesions in women more than 30 years and it is more sensitive than VIA or cytology. Sensitivity 66% to 95%.
A new test- care HPV (Qlagen, INC): A new test has been developed and field tested for use in low resource settings. This test can detect DNA from 14 cancer causing types of HPV with test results available in about 2.5 hrs, without extensive lab facilities. However, both care HPV and other high cost tests are designed to test many samples at the same time.
Visual inspection of cervix after acetic acid (VIA) application: it has sensitivity comparable to or greater than that of cytology- 41–79%, It requires simple equipment and relatively brief training and can be performed by middle level health personnel, results are immediately available and if indicated treatment can be provided at the same visit (screen & treat program), thus reducing the loss to patient follow up.
VIA positive: There is a moderately opaque, sharply bordered, wide band of aceto white staining around the cervical os, touching the squamocolumnar junction (Figure 6). There is mild aceto white staining of the immature metaplastic epithelium extending onto the endocervical polyp.
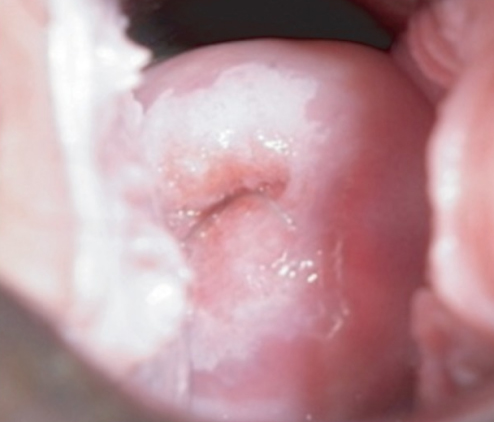
Figure 6: Positive result (aceto white lesion) of visual inspection after the application of acetic acid.
A screening programme is considered successful when it covers 80% of target population; when more cases of in situ lesions than invasive lesions are detected; when periodic rescreening is undertaken and in each such screening less number of cancer cases are detected; when mortality due to cervical cancer declines. Properties and characteristics of different screening methods are discussed in the table 3.
Table 3: Properties and characteristics of different screening methods.
|
screening test
|
Sensitivity
|
Specificity
|
characteristics
|
disadvantages
|
|
Cytology
|
Low to moderate
44-78%
|
high 91-96%
|
Requires health based infrastructure; laboratory, training, subjective
|
Expensive, requires infrastructure and training; 2/3rd of false negatives are due to errors of sampling; 40-50% cancers in cases screened within 5 years; 15-42% fail to obtain evaluation; 10-18% are never notified
|
|
HPV DNA
|
High (66-100%)
|
Moderate (61-90%)
|
Lab based, objective, reproducible, expensive
|
--
|
|
Visual inspection
|
--
|
--
|
Low technology, low cost, linkage to immediate treatment
|
--
|
|
VIA
|
Moderate 67-79%
|
Low 49-86%
|
Suitable for low resource settings
|
--
|
|
VILLI
|
Moderate high 78-98%
|
Moderate 73-93%
|
--
|
--
|
One of the prerequisites for effective screening is the availability of a suitable cervical screening test that has adequate sensitivity and specificity for detection of precancerous lesions and that yields reproducible results. Such a test should be cheap, simple, and easy to apply; without side effects or complications; as painless as possible; and socio culturally acceptable.
Cervical cancer screening –Indian perspective
As India is a diverse country with varied scenarios. With 75% of population in rural or low resource setting and 25% of population is in urban high resource setting, a uniform strategy can not be implemented. Any new program needs to be integrated in the existing healthcare services. At present there are no organized screening programs available. Recently launched National program for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS, Ministry of Health & Family Welfare - Government of India), has among its major objectives, universal cancer control through opportunistic screening of woman above the age of 30 years.
Cytology based screening programs are difficult to implement and sustain, because of lack of supplies, trained personnel, equipment, quality control, health care infrastructure and effective follow up procedures. Thus creating, staffing, and maintaining cytology labs are not feasible. Even where it is feasible, cytology has got low sensitivity (53% in high resource countries and 26% in low resource countries), which means that the test misses a good number of precancers and cancers. In India the vast majority of woman has never been screened and would be fortunate to have one or two opportunities for screening in their life times. Even then often they would be unable to return for treatment.
In India universal vaccination can have substantial effect in reducing the morbidity of cervical cancer, wherein an organization screening program may not exist for all women in near future.
Evolutions of cervical cancer screening intervals are 1940–1989- Annual PAP for all women & linkage to annual health checkup; 1987- Walton commission (British Columbia) cytology screening every 3 years; 1989- AMA, ACOG, AMWA annually, starting at sexual activity or 18 yrs old. After 3 negative smears testing may be done less frequently, longer intervals based on the absence of risk factors; 2013- cervical cancer screening guidelines for average risk woman.
2013 Cervical cancer screening guidelines for average risk woman (Endorsed by ACS, ASCCP, ASCP & USPSTF)
1st time all the three organizations involved in cervical cancer prevention & USPSTF have endorsed equivalent guidelines (Table 4).
Table 4: 2013 cervical cancer screening guidelines.
|
Below 21 yrs
|
No screening as at this age invasive cancer is rare, HPV infection is transient & spontaneous regression of low grade lesions common
|
|
21- 29 yrs
|
cytology alone every 3 yrs
|
|
30 – 65 yrs
|
Acceptable – cytology alone every 3 yrs, Preferred is cytology + HPV every 5yrs
|
|
More than 65 yrs
|
No screening following 3 consecutive negative prior screens in last decade, as HPV risk is less & even present unlikely to progress to invasive stage during the remaining life time.
|
|
HIV positive & Immune suppressed
|
Annually
|
|
After TAH (if cervix removed)
|
No screening
|
|
After HPV vaccination
|
Screening as per age specific guidelines
|
In low- and middle income countries, because of the high cost of setting up screening programmes based on cytology, coverage of screening is very low and alternative screening methods are needed. In addition, follow-up of a positive cytology test with colposcopy and biopsy requires resources and skilled personnel that are largely lacking in many countries. Other bottlenecks include the need for referral to distant health facilities for diagnostic and treatment services, and the long waiting times before cytology results are available. An alternative approach to diagnosing and treating CIN is to use a ‘screen-and-treat’ approach in which the treatment decision is based on a screening test, and not on a histologically confirmed diagnosis of CIN2+, and treatment is provided soon or, ideally, immediately after a positive screening test.
Screen-and-treat programme or single visit approach for cervical cancer
The goal of a screen-and-treat programme for cervical cancer is to reduce cervical cancer and related mortality with relatively few adverse events. The programme must include a screening test or strategy (sequence of tests) and be linked to appropriate treatments for CIN, and also provide referral for treatment of women with invasive cervical cancer. Common screening tests that are widely used include tests for human papillomavirus (HPV), cytology (Pap test), and unaided visual inspection with acetic acid (VIA). These tests can be used as a single test or in a sequence. When using a single test, a positive result indicates the need for treatment. When using a sequence of tests, women who test positive on the first test receive another test and only those who test positive on the second test are treated. Women with a positive first screening test followed by a negative second screening test are followed up.
Available treatments include cryotherapy, large loop excision of the transformation zone (LEEP/ LLETZ), and cold knife conization. This guideline provides recommendations for strategies for a screen-and-treat programme. It builds upon the existing recommendations for the use of cryotherapy to treat CIN [9, 10] and on the new WHO guidelines for treatment of cervical intraepithelial neoplasia 2–3 and glandular adenocarcinoma in situ [11], which is being published concomitantly with these present guidelines. When developing the guideline, the Guideline Development Group (GDG) considered that countries currently providing screen-and-treat programmes may be uncertain about which strategy to use.
Recommendations
The recommendations were developed by comparing the benefits and harms of different screen-and-treat strategies. For countries where a cervical cancer prevention and control programme already exists, these recommendations were developed to assist decision-makers to determine whether to provide a different screening test followed by a different treatment instead, or to provide a series of tests followed by an adequate treatment. For countries where such a programme does not currently exist, these recommendations can be used to determine which screening test and treatment is to be provided.
The recommendations and background information about the various screening tests and treatments are also available in an updated version of comprehensive cervical cancer control: a guide to essential practice (C4-GEP) [12]. The C4-GEP was originally published in 2006 by the World Health Organization (WHO) to assist clinicians and programme managers to diagnose and treat CIN in order to prevent and control cervical cancer.
In 2009, WHO committed to updating the C4-GEP as specific aspects deserved the development of new recommendations. In particular, new evidence had become available regarding the use of cryotherapy for CIN (a new guideline was completed in 2011 [10]); treatment of histologically confirmed CIN2+ (a new guideline is being published concomitantly with this present one [11]); and strategies for screening and treatment of precancerous cervical lesions (the subject of this present guidance). In addition, there is a new awareness that when making recommendations for screen-and-treat strategies, the consequences of treating or not treating women after positive or negative screening results should also be considered. Typically, the selection of a screening test is based on its accuracy, which is determined by calculating the sensitivity and specificity of the test, and recommendations are based on that evidence. However, these data, do not address the consequences of screening and treating women. When deciding on a screen and-treat strategy, it is critical to consider the downstream consequences after treatment (for a positive test) or no treatment (for a negative test), such as cervical cancer and related mortality, recurrence of CIN2+, adverse effects of treatment (and overtreatment), and use of resources. These recommendations are based on evidence about the diagnostic accuracy of each of the screening tests, together with evidence about the benefits and harms of treatments.
This guideline provides recommendations for strategies for a screen-and-treat programme. It builds upon the existing WHO guidelines: Use of cryotherapy for cervical intraepithelial neoplasia (published in 2011) and on the new WHO guidelines for treatment of cervical intraepithelial neoplasia 2–3 and glandular adenocarcinoma in situ (being published concomitantly with these present guidelines). This guideline is intended primarily for policy-makers, managers, programme officers, and other professionals in the health sector who have responsibility for choosing strategies for cervical cancer prevention, at country, regional and district levels. This guideline provides nine recommendations for screen-and-treat strategies to prevent cervical cancer.
Areas for future research include screen-and-treat strategies using a sequence of tests (e.g. HPV test followed by VIA); screen-and-treat strategies in women of HIV-positive status; and measurement of important health outcomes following a screen-and-treat strategy (Table 5).
Table 5: Screen-and-treat strategies- involve treatment with cryotherapy, or LEEP when the patient is not eligible for cryotherapy.
|
· Use a strategy of screen with an HPV test and treat, over a strategy of screen with VIA and treat. In resource-constrained settings, where screening with an HPV test is not feasible, the panel suggests a strategy of screen with VIA and treat.
· Use a strategy of screen with an HPV test and treat, over a strategy of screen with cytology followed by colposcopy (with or without biopsy) and treat. However, in countries where an appropriate/high-quality screening strategy with cytology followed by colposcopy already exists, either an HPV test or cytology followed by colposcopy could be used.
· Use a strategy of screen with VIA and treat, over a strategy of screen with cytology followed by colposcopy (with or without biopsy) and treat. The recommendation for VIA over cytology followed by colposcopy can be applied in countries that are currently considering either programme or countries that currently have both programmes available.
· Use a strategy of screen with a HPV test and treat, over a strategy of screen with an HPV test followed by colposcopy (with or without biopsy) and treat.
· Use either a strategy of screen with an HPV test followed by VIA and treat, or a strategy of screen with an HPV test and treat.
· Use a strategy of screen with an HPV test followed by VIA and treat, over a strategy of screen with VIA and treat.
· Use a strategy of screen with an HPV test followed by VIA and treats, over a strategy of screen with cytology followed by colposcopy (with or without biopsy) and treat.
· Use a strategy of screen with an HPV test followed by VIA and treat, over a strategy of screen with an HPV test followed by colposcopy (with or without biopsy) and treat.
|
As shown in the figure 7, a decision-making flowchart has been developed that will assist programme managers to choose one of the suggested strategies, depending on the context where it will be implemented.
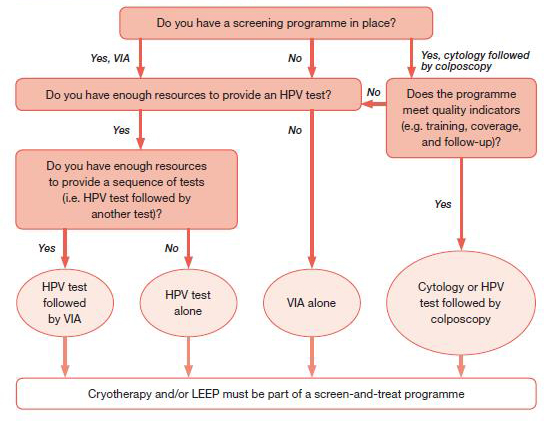
Note: each light-pink bubble refers to one strategy in Annex 3 (for women of negative or unknown HIV status) or Annex 4 (for women of HIV-positive status or unknown HIV status in areas with high endemic HIV infection.
Figure 7: Decision-making flowchart for programme managers.
Conclusions
Launching community based low intensity cervical screening in combination with awareness campaign and monitoring system should be the priority of the cervical cancer control program. Vaccines against HPV infections are now available, but are very expensive. More qualitative studies are required looking at the psycho-social and cultural barriers faced by women in different parts of the country when it comes to taking steps to avoid getting HPV infection, going for medical check-ups, screening and following treatment plans. WHO issues guidelines for policy-makers, managers, programme officers, and other professionals in the health sector who have responsibility for choosing strategies for cervical cancer prevention will be helpful.
Conflicts of interest
Authors declare no conflicts of interest.
References
1. Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide, IARC Cancer Base No.5, version 2.0, Lyon: IARC Press; 2004.
2: Kurkure AP, Yeole BB. “Social inequalities in cancer with special reference to South Asian countries”, Asian Pacific Journal of Cancer Prevention 7(1):36-40.
3. Waggoner SE. “Cervical Cancer”. Lancet 361:2217-2225.
4. Joy T, Sathian B, Bhattarail C, Chacko J. Awareness of cervix cancer risk factors in educated youth: a crosssectional, questionnaire based survey in India, Nepal, and Sri Lanka. Asian Pac J Cancer Prev. 2011; 12(7):1707-1712.
5. Lazcano-Ponce EC, Castro R, Allen B, Nájera P, Alonso de Ruíz PA, et al. Barriers to early detection of cervical-uterine cancer in Mexico. J Women’s Hlth. 1999; 8(3):399-408.
6. Bradley J, Coffey P, Arrossi S, Agurto I, Bingham A, et al. Women’s perspectives on cervical screening and treatment in developing countries: experiences with new technologies and service delivery strategies. Women Hlth. 2006; 43:103-121.
7. The News Letter of NCRP (2011) NCRT-ICMR. Cancer Registry Abstract Crab 16(1):1-24
8. Goodman A, Wilbur DC, N Engl J Med. 2003; 349: 1555-1564
9. Santesso N, Schünemann H, Blumenthal P, De Vuyst H, Gage J, et al. World Health Organization Guidelines: Use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2012; 118(2):97–102.
10. WHO guidelines: use of cryotherapy for cervical intraepithelial neoplasia. Geneva, World Health Organization, Department of Reproductive Health and Research, 2011 (http://www.who.int/reproductivehealth/publications/cancers/9789241502856/en/, accessed on 24 October 2013).
11. WHO guidelines for treatment of cervical intraepithelial neoplasia 2–3 and glandular adenocarcinoma in situ: Cryotherapy, large loop excision of the transformation zone (LEEP/ LLETZ), and cold knife conization. Geneva, World Health Organization (in process).
12. Comprehensive cervical cancer control: a guide to essential practice (C4-GEP). Geneva, World Health Organization, Department of Reproductive Health and Research and Department of Chronic Diseases and Health Promotion, 2006. (http://www.who.int/reproductivehealth/publications/cancers/9241547006/en/, accessed on 20 August 2013).