Letter to theEditor
2015
September
Volume : 3
Issue : 3
Tonsillar enlargement with growth hormone therapy
Babulreddy Hanmayyagari
Pdf Page Numbers :- 105-106
Babulreddy Hanmayyagari1
1Department of Endocrinology, Krishna Institute of Medical Sciences, Kondapur, Hyderabad -500084, Telangana, India
*Corresponding author: Dr. H. Babul Reddy, Department of Endocrinology, Krishna Institute of Medical Sciences, Kondapur, Hyderabad - 500084, Telangana, India. Mobile: +91 9985661434; Email: babulreddy78@gmail.com
Received 14 May 2015; Revised 16 June 2015; Accepted 23 June 2015; Published 30 June 2015
Citation: Babulreddy H. Tonsillar enlargement with growth hormone therapy. J Med Sci Res. 2015; 3(3):105-106. DOI: http://dx.doi.org/10.17727/JMSR.2015/3-020
Copyright: © 2015 Babulreddy H. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Full Text
A 14-year-old boy presented to the ENT department with shortness of breath, stridor and dysphagia since 4 months and was diagnosed to have tonsillar enlargement & surgery was planned. He was referred to department of endocrinology as he is a known patient of hypopituitarism.
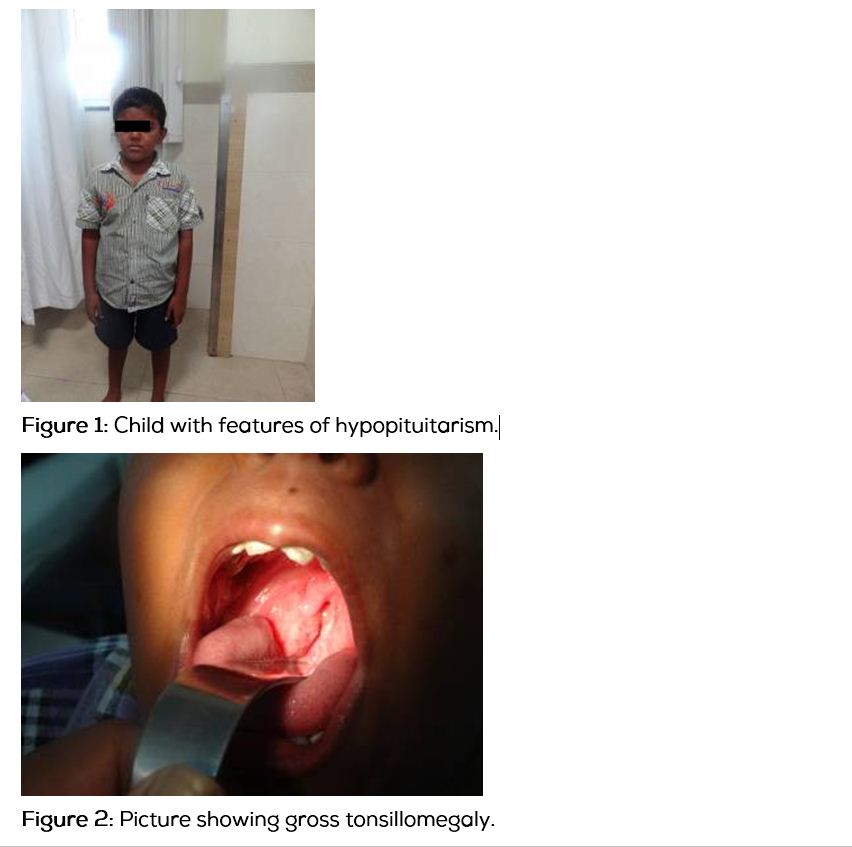
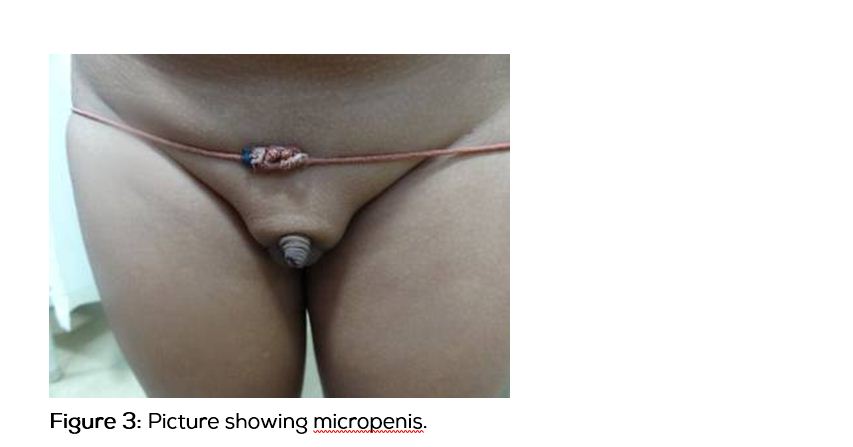
This boy was diagnosed to have hypopituitarism 1 year ago, and has been on human growth hormone and thyroxine replacement for the past 8 months. The above symptoms started 4 months after initiation of treatment and are progressive in nature. There is no history of adrenal insufficiency or development of secondary sexual characters. On examination, his height was 117cm US/LS-0.9, SDS was -7, weight was 25 kg and head circumference was 51cm. He had mild facial hypoplasia, depressed nasal bridge, doll like facies (Figure1), high pitched voice, central obesity, micropenis (Figure 2) and a prepubertal sexual maturity rating. Oral examination revealed gross tonsillar enlargement (Figure 3). His vitals were stable. Systemic examination was normal.
His laboratory investigations were as follows: Hemoglobin-13gm%, total leukocyte count-5600/cumm , ESR-15, FBS-89mg%, sr.creatinine-0.8mg/dl, T3-1.2 ng/ml, T4-8.4 mcg/ml, TSH-2.7 miU/ml, serum cortisol-5.7 mcg/dL, bone age- 9 years, MRI brain-pituitary hypoplasia. Child subsequently underwent tonsillectomy under steroid coverage.
Even though human growth hormone (hGH) has been used since 1958, it was only in 1985, that there was a switch from cadaveric pituitaries to recombinant hGH (rhGH) obtained from DNA-recombinant techniques, after the reports of Creutzfeldt-Jakob disease in hGH recipients [1]. Creutzfeldt- Jakob disease has never been reported with the use of rhGH.
Since 1985, rhGH has been approved not only for GHD but also for a series of non-GH deficient disorders, such as chronic renal failure, Turner syndrome, small-for-gestational age, Prader-Willi syndrome, idiopathic short stature, SHOX gene haploinsufficiency, Noonan syndrome [2] and adult growth hormone deficiency [3].
Side effects of rhGH replacement therapy include rash and pain at the injection site, tonsillar enlargement, prepubertal gynecomastia, arthralgia, edema, benign intracranial hypertension, insulin resistance, progression of scoliosis, and slipped capital femoral epiphysis. Since GH stimulates cell multiplication, development of neoplasms is a concern.
IGF-I-mediated hypertrophy of the tonsillar/adenoid tissue [4] has been reported to cause obstructive sleep apnea in Prader-Willi syndrome (PWS) [5] and acromegaly patients. Here the tonsillar enlargement was started after starting GH therapy and raise in the levels of growth hormone is a possible contributing factor for this. However, gross tonsillar enlargement requiring tonsillectomy is rare with growth hormone therapy and is hence being reported here.
Conflict of interest
The author declares no conflict of interest.
References
1. Fradkin JE. Creutzfeldt-Jakob disease in pituitary growth hormone recipients. Endocrinologist. 1993; 3:108–114.
2. Richmond E, Rogol AD. Current indications for growth hormone therapy in children and adolescents. Endocr Dev. 2010; 18:92–108.
3. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. JCEM. 2011; 91(5):1621–1634.
4. Cadieux RJ, Kales A, Santen RJ, Bixler EO, Gordon R. Endoscopicfindings in sleep apnea associated with acromegaly. J Clin Endocrinol Metab. 1982; 55(1):18–22.
5. Miller J, Silverstein J, Shuster J, Driscoll DJ, Wagner M. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab. 2006; 91(2):413–417.