Full Text
Introduction
The diagnosis of a bone tumor is dependent on an evaluation of clinical, radiologic, and pathologic features. In most cases, the pathologist can render an accurate diagnosis based on histopathologic features. In some cases, however, a variety of ancillary studies, especially immunohistochemistry (IHC), has proven to be more helpful in distinguishing between entities that share similar morphologic features or are of uncertain histogenesis. Immunohistochemistry (IHC) provides the histological identification of antigenic factors in cells not otherwise evident in routinely stained histological sections. It is more helpful in elucidating the histogenesis of the cell population, but less helpful in distinguishing between reactive, benign and malignant lesions. This review will focus on frequently used diagnostic immunomarkers in selected primary bone tumors based on the World Health Organization tumor classification of 2005.
Osteogenic tumors
Osteosarcoma: osteosarcoma is the second most common primary bone tumor, the first being multiple myeloma. The diagnosis of osteosarcoma is generally based on radiologic and morphologic features. The primary utility of IHC is to exclude other diagnostic possibilities, such as metastatic sarcomatoid carcinoma, synovial sarcoma, ES/PNET and MFH, especially in biopsies in which production of osteoid matrix by the tumor cells is not evident. The two markers which aid in the diagnosis of difficult cases are osteonectin and osteocalcin. Osteocalcin is produced exclusively by bone forming cells and therefore are specific marker for OS. It exhibits 70% sensitivity and 100% specificity. In bone tumors it is mainly used in differential diagnosis of OS (1, 2). Osteonectin is a secreted glycoprotein that modifies cell–extracellular matrix interactions. It is expressed in normal tissues undergoing proliferation and remodelling, such as during morphogenesis, wound healing and angiogenesis. Osteonectin is produced by bone cells. Over expression by tumor cells has been reported in various malignancies, including breast carcinoma, melanoma, glioma, meningioma, osteosarcoma and malignant fibrous histiocytoma. Because of its wide expression, osteonectin lacks specificity for bone tumors. It exhibits 90% sensitivity and 54% specificity reported for osteonectin. It is, therefore, inferior to osteocalcin as a marker of osteoblastic differentiation [1-3].
Osteoma, osteoid osteoma and osteoblastoma: Osteoma, osteoid osteoma and osteoblastoma are benign osteogenic tumors. The diagnosis of these entities generally relies on radiologic and morphologic features. Immunohistochemistry offers little help in the differential diagnosis.
Cartilaginous tumors
Chondrosarcoma: This malignant tumor of bone is characterized by the presence of hyaline cartilage production. Immunohistochemically, chondrosarcoma cells are positive for S-100, estrogens receptors [5] and Sox9 [9]. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors [4, 10, 11]. Mesenchymal chondrosarcoma presents multiple challenges. Diagnostic pitfalls include inadequate biopsy samples, which may result in sample error. Sox9 has been proposed as a unique marker for mesenchymal chondrosarcoma which may improve diagnostic specificity. Thorough histological examination, when coupled with immunohistochemical findings, is helpful in arriving at a correct diagnosis [8, 10, 11].
Clear cell variant of chondrosarcoma is another entity where IHC is sometimes required especially in small biopsies. The negative markers are of greatest use in this circumstance. The most useful negative markers to distinguish from metastatic renal cell carcinoma are absence of co-expression of keratin and vimentin, and CD10. Clear cell chondrosarcomas strongly co-expresses Sox9, S-100 and type II collagen. IHC is also helpful in the distinction of chondrosarcoma from chordoma, which expresses EMA, cytokeratins, and HBME-1 [9].
Chondroblastoma: Chondroblastoma is a benign bone tumor, characterized by a proliferation of immature cartilage cells with foci of cartilage matrix formation.IHC is not required in the diagnosis of this entity. IHC is useful in the distinction of chondroblastoma from non-cartilage lesions that contain giant cells, such as giant-cell tumor and giant cell reparative granuloma, which do not express S-100. Their mononuclear cells are usually strongly positive for histiocytic markers such as CD68 and alpha-1-chymotrypsin [9].
Osteochondroma, chondroma, chondromyxoid fibroma and synovial chondromatosis: The diagnosis of these tumors generally relies on radiologic and histological features. IHC has little or no value in the differential diagnosis of this group of tumors.
Fibrohistiocytic tumors
Malignant fibrous histiocytoma (MFH): in MFH vimentin is strongly positive in tumor cells, and SMA and CD68 are usually positive. Immunomarkers are of limited value in the diagnosis of MFH of bone. However, they are useful to rule out other spindle cell tumors that may resemble MFH, such as osteosarcoma, leiomyosarcoma, metastatic melanoma and neurilemmoma [1] (Table 1).
Table 1: Immunohistochemistry for differential diagnosis of malignant fibrous histiocytoma:
|
vimentin
|
SMA
|
CD68
|
S-100
|
OC
|
AP
|
HMB-45
|
MFH
|
+
|
+/-
|
+
|
-
|
-
|
-
|
-
|
LMS
|
+
|
+
|
-
|
-
|
-
|
-
|
-
|
MPNST
|
+
|
-/+
|
-
|
+
|
-
|
-
|
-
|
Fibroblastic OS
|
+
|
-
|
-
|
-
|
+
|
+
|
-
|
Spindle cell melanoma
|
-
|
-
|
-
|
+
|
-
|
-
|
+
|
Metastatic sarcomatoid
carcinoma
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
Abbreviations: MFH= Malignant fibrous histiocytoma; LMS= Leiomyosarcoma; SMA= smooth muscle actin; OC= osteo calcium; AP= alkaline phosphatase.
Benign fibrous histiocytoma of bone: Benign fibrous histiocytoma of the bone is generally diagnosed on the basis of morphology. No specific marker is employed in its diagnosis.
Haemopoitic tumors
Plasma cell myeloma: myeloma cells are usually positive for kappa or lambda light chains (usually one markedly more than the other), CD38 9plasma cells, CD79a, CD 138, variable EMA, variable CD10. They are negative for CD45/LCA, CD19 and CD 20. The main differential diagnosis are plasmacytoid lymphoma (positive for CD19, CD20 and CD22 and negative for CD38, CD 79a, CD138 and EMA) ;and poorly differentiated carcinoma ( positive for pancytokeratin) [1].
Fibrogenic tumors
Desmoplastic fibroma of bone and fibrosarcoma of bone are the tumors in this category. IHC has little or no role in the diagnosis of these entities.
Vascular tumors
Hemangiomas and related lesions are benign vasoformative tumors or developmental conditions of endothelial origin. Epithelioid hemangioendothelioma, a neoplasm of intermediate-grade malignancy, and angiosarcomas are composed of cells that show endothelial differentiation. All the vascular lesions are usually positive for at least one of the endothelial markers CD31, CD34 and FVIII. In addition, epithelioid vascular tumors may also express cytokeratins, EMA and Fli-1. Distinction of these entities from non-endothelial tumors relies on the positivity for CD31, CD34 and FVIII [1, 9].
Myogenic, lipogenic, neural and epithelial tumors
Smooth muscle tumors: of bone are rare spindle cell tumors with smooth muscle differentiation. A metastasis from a distant site, especially the uterus, needs to be excluded prior to making a diagnosis of primary leiomyosarcoma. Expression of smooth muscle markers such as SMA, muscle specific actin and desmin is consistently present in these tumors [1] Lipoma and liposarcoma of bone are tumors from adipocytes or with adipocytic differentiation. The tumor cells are positive for S-100. However, the diagnosis of these two entities generally relies on the radiologic and histological features. Neurilemmoma (schwannoma) is a benign tumor of schwann cell origin arising within bone. The tumor cells are strongly and diffusely positive for S-100. IHC is of use in differentiating it from leiomyoma and well-differentiated leiomyosarcoma, especially in small biopsies [1, 9].
Adamantinoma is a rare malignant primary bone tumor with epithelial and mesenchymal elements. Pancytokeratin is positive in epithelial and spindle cells. Epithelial cells also show co-expression of cytokeratin, EMA and vimentin.
Giant cell tumor (GCT) is a benign, locally aggressive tumor composed of a combination of round-to-oval mononuclear cells and uniformly distributed osteoclast-type giant cells with nuclei similar to those of the mononuclear cells. The osteoclast-type giant cells are positive for CD68 and common muscle actin. Both the mononuclear cells and the giant cells are positive for vimentin, alpha-1-antitrypsin, alpha-1-antichymotrypsin, tartrate-resistant acid phosphatase (TRAP) and osteocalcin [14] and ER receptor antigen [15] but not for EMA. Multinucleated giant cell-containing tumors and pseudotumors of bone represent a heterogeneous group of benign and malignant lesions. Differential diagnosis can be challenging, particularly in instances of limited sampling. P63 expression seems to differentiate between giant cell tumor of bone and central giant cell granuloma since the latter does not express P63. Other benign and malignant giant cell-containing lesions express P63, decreasing its specificity as a diagnostic marker, but a strong staining was seen, except a case of chondroblastoma, only in giant cell tumor of bone. Clinical and radiological confrontation remains essential for an accurate diagnosis [7].
Ewing’s sarcoma/primitive neuroectodermal tumor
ES/PNETs are round-cell sarcomas that show varying degrees of neuroectodermal differentiation. These are rare tumors, predominantly of young adults, that show the same t (11;22)(q24;q12) translocation and EWS-FLi-1 gene fusion. CD99 is expressed in almost all ES/PNETs in a characteristic membranous pattern, although it is not specific Vimentin stains most ES/PNET cells. Fli-1 and neural markers such as NSE, chromogranin and synaptophysin, are frequently expressed by ES/PNETs [1, 9, 12, 13] . Occasionally, ES/PNETs have been shown to stain focally for cytokeratins. The main differential diagnoses for ES/ PNET are other small-round-blue-cell tumors, which include neuroblastoma, rhabdomyosarcoma, mesenchymal chondrosarcoma, lymphoma/ leukemia, desmoplastic small round-cell tumor, small cell carcinoma (poorly differentiated neuroendocrine carcinoma), poorly differentiated synovial sarcoma, small cell melanoma and small cell osteosarcoma [12, 16]. Table 2 and figure illustrates a useful initial panel of immunomarkers for these tumors and includes markers for lymphoid, neural, neuroendocrine, muscle and epithelial antigens.
Table 2: Immunomarker panel for small round cell tumors of bone
Ewings sarcoma
|
CD99, Fli-1, NSE, CGA, SYP
|
Metastatic NB
|
NSE, SYN, CGA
|
Metastatic RMS
|
Desmin, SMA, Myogenin
|
Lymphoma/Leukemia
|
CD45, CD3, CD5, CD20, Pax5
|
Small cell OS
|
Osteocalcin, osteonectin
|
Mesenchymal chondrosarcoma
|
Sox 9, S-100, vimentin
|
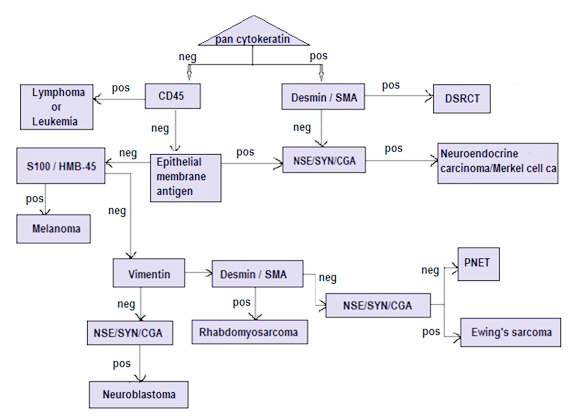
Figure 1: Flow chart for diagnosis of small round cell tumors by immunohistochemistry.
Abbreviations: SMA: smooth muscle actin; NSE: neuron specific enolase; SYN: synaptophysin; CGA: chromogranin; PNET: primitive neurectodermal tumor.
Undifferentiated non-small-cell tumors [1, 9]
Some tumors may be so undifferentiated that routine microscopy fails to suggest their histogenesis. In this situation an initial broad panel is helpful and is listed in Table 3. The results from this initial panel will determine which additional immunomarkers are needed to establish a diagnosis. For example, a tumor that is positive for CD45 but negative for cytokeratin and vimentin is likely to be of lymphoid origin. A lymphoma panel can then be applied to render a more specific diagnosis. A tumor that is strongly positive for cytokeratin but negative for CD45 and vimentin is most likely to be of epithelial origin and may require cytokeratin subsets for more precise identification. Similarly, a tumor that is positive for vimentin but negative for CD45 and cytokeratin is most likely to be of mesenchymal origin, which requires more specific mesenchymal markers for identification.
Table 3: Initial immunohistochemical panel for undifferentiated non-small-cell carcinoma.
Markers
|
Epithelial tumors
|
Mesenchymal tumors
|
Lymphoid tumor
(T or B cell)
|
CD45
|
-
|
-
|
+
|
PCK
|
+
|
-/+
|
-
|
Vimentin
|
-/+
|
+
|
-
|
Abbreviations: PCK= pancytokeratin; + = positive; - = negative; -/+ = sometimes positive.
Notochordal tumors [1, 9]
Chordoma is a neoplasm arising from embryonic notochordal remnants of the neural axis. Chordoma is positive for subsets of cytokeratins (CK5, CK8, CK18 and CK 19), S-100, glial fibrillary acidic protein (GFAP), EMA, vimentin, HBME-1, NSE and, occasionally, for synaptophysin but not for chromogranin. IHC is especially useful in the diagnosis of chordoma when only small biopsy specimens are available with limited material for morphologic observation. Histologically, chordoma may be confused with mucinous carcinoma, myxoid chondrosarcoma, renal cell carcinoma, extra-skeletal myxoid chondrosarcoma and other clear-cell neoplasms. Expression of cytokeratin subsets is useful in differentiating chordomas from chondrosarcomas. Cytokeratins are rarely expressed by skeletal chondrosarcomas, although CK8 and, to a lesser extent, CK19 can be expressed by extra-skeletal myxoid chondrosarcoma. In addition, GFAP and galectin-3 are usually positive in chordoma but negative in chondrosarcoma. HBME-1, a marker for mesothelial cells, is strongly expressed by chordoma and skeletal chondrosarcoma but not by renal (positive for CD10 and vimentin) or colorectal carcinoma. Pituitary adenomas should be included in the differential diagnosis of chordoma. They express all neuroendocrine markers and lack expression of CK8 and CK19.
Tumor-like lesions [1, 9]
Langerhans cell histiocytosis is a neoplastic proliferation of Langerhans cells, which are found in the epidermis, cervix, vagina, stomach and esophagus. Langerhans cells have a dendritic morphology, contain Birbeck granules demonstrated by electron microscopy, and play a key role in antigen presentation. They have a characteristic immunophenotype, which includes expression of membrane based CD1a and S-100 in both a nuclear and a cytoplasmic pattern [1]. These cells typically fail to express CD45. The typical immunophenotype for langerhans cell histiocytosis is CD1a+/S100+/CD21−/CD35−/CD86− [1]. The main differential diagnosis of Langerhans cell histiocytosis is from other histiocytic and dendritic cell neoplasms (Table 4). Follicular dendritic cell tumors/sarcomas have a dendritic morphology and desmosomes. They have an immunophenotype of CD21+/CD35+/CD1a− and demonstrate low levels of lysosomal enzymes [1, 9]. Interdigitating dendritic cell sarcoma or reticulum cell sarcoma is a spindle cell neoplasm with the phenotype of interdigitating dendritic cells, which are found in the T-zone of lymph nodes. The neoplastic cells have a dendritic morphology, show low levels of lysosomal enzymes and have an immunophenotype of S-100+/CD1a−/CD21−/CD35−/ CD86+. Erdheim–Chester disease (ECD) is a rare histiocytosis, characterized by infiltration of the skeleton and viscera by lipid-laden histiocytes, leading to fibrosis and osteosclerosis. The tumor cells express immunomarkers of the macrophage lineage (CD68, lysozyme, Mac387, CD4, antichymotrypsin and antitrypsin). They are usually negative for CD1a and S-100, which helps to distinguish this entity from Langerhans cell histiocytosis and Rosai–Dorfman disease. Rosai–Dorfman disease (sinus histiocytosis with massive lymphadenopathy) is a rare disorder characterized by proliferation of histiocytic cells within the sinuses of lymph nodes as well as in various extra-nodal locations. The etiology is unknown. Molecular studies have found no evidence of clonal rearrangement, which implies that this disease is reactive rather than neoplastic in nature. It commonly presents as painless enlargement of lymph nodes in the neck, with fever. Most cases occur in the first or second decade of life and have a predilection for the black population. It may occur in other organs of the body, such as skin, central nervous system, kidney, digestive tract and bone. Skeletal involvement is relatively uncommon and is often secondary to extra-skeletal lesions. Typically, the cells are positive for CD68, S-100 protein and negative for CD1a (Table 4).
Table 4: Immunohistochemical panel for histiocytic lesions
Markers
|
CD68
|
S-100
|
CD1a
|
LCH
|
-
|
+
|
+
|
ECD
|
+
|
-
|
-
|
RDD
|
+
|
+
|
_
|
Abbreviations: LCH= langerhans cell histiocytosis; ECD= Erdheim–Chester disease; RDD= Rosai–Dorfman disease
Metastatic tumors involving bone (Tables 5 and 6)
Bone is the third most common metastatic site after lung and liver. Metastatic carcinomas are the most common tumors affecting bones. More than 90%of these metastatic carcinomas are from breast, lung, prostate, kidney and thyroid gland. Clinical and radiographic evaluation can identify the primary site in about 80% of cases. Normally, metastatic tumors retain the morphologic features of the original tumor. However, IHC is helpful in cases in which the primary site is occult. For example, estrogen receptor, progesterone receptor and gross cystic fluid protein are positive in breast carcinoma, thyroid transcription factor-1 (TTF-1) in lung carcinoma, prostatespecific antigen (PSA) in prostatic carcinoma, renal cell carcinoma marker (RCCMA) and CD10 in renal carcinoma, and thyroglobulin in thyroid carcinoma [6].
Table 5: Metastatic bone tumors, unknown primary – stepwise approach (6)
Step 1 : identify broad cancer type
1. Carcinoma
2. Melanoma
3. Lymphoma/leukemia
4. Sarcoma
|
Pan CK, CK7, CK20,CK5,EMA
S-100,HMB-45,Melan A
CD45,CD20,CD3,CD138,CD30 etc.,
Vimentin,actin,desmin,s-100, C-kit etc.,
|
Step 2 : If carcinoma then identify subtype
1. Adenocarcinoma
2. Sq. Cell carc
3. Transitional cell carcinoma
4. RCC
5. Liver
6. Thyroid
7. Adrenal
8. Germ cell tumor
9. Mesothelioma
10. Neurendocrine carcinoma
|
CK7,CK20,PSA, other adenocar. markers CK5,p63
CK7,CK20,urothelin
RCC,cd10,pax 8, napsin A
Hepar-1,CD 10,GLYCIPAN 3
TTF-1,thyroglobulin, pax-8
Melan A, inhibin
OCT-4,PLAP,HCG,AFP
Calretinin, mesothelin, WT-1
Chromogranin, CD 56, synaptophysin, TTF-1
|
Step 3: if adenocarcinoa, then predict possible primary site
|
Table 6
|
Abbreviations: EMA= epithelial membrane antigen; TTF= Thyroid transcription factor; PLAP= Placental alkaline phosphatase
Table 6: Prediction of primary site of adenocarcinoma by using IHC
|
prostate
|
lung
|
breast
|
Ovary
serous
|
Ovary
mucinous
|
pancreas
|
stomach
|
colon
|
PSA
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
TTF-1/
Napsin
|
-
|
+
|
-
|
-
|
-
|
-
|
-
|
-
|
GCDFP-15
|
-
|
+/-
|
-
|
-
|
-
|
-
|
-
|
-
|
WT-1
|
-
|
-
|
-
|
+
|
-
|
-
|
-
|
-
|
PAX-8
|
-
|
-
|
-
|
+
|
-/+
|
|
|
|
ER
|
-
|
-
|
+/-
|
+/-
|
-/+
|
-
|
-
|
-
|
CA-125
|
-
|
-/+
|
-/+
|
+
|
-/+
|
+/-
|
-
|
-
|
CK-7
|
-
|
+
|
+
|
+
|
-/+
|
+
|
+/-
|
+/-
|
CDX-2/
CK 20
|
-
|
-
|
-
|
-
|
-/+
|
-/+
|
-
|
-
|
Abbreviations: PSA= prostate specific antigen; TTF= Thyroid transcription factor; GCDFP-15= Gross cystic disease fluid protein 15 (GCDFP-15); + = positive; - = negative; -/+ = sometimes positive.
Conclusion
IHC plays an important role in the diagnosis of some primary bone tumors, especially in distinguishing primary bone tumors from metastatic tumors and in the categorization of small-round-blue-cell tumors. IHC is required only in 3-5% of bone tumors. Use of two positive and two negative markers is ideal to improve the specificity. Choice of markers depends upon what tumors are logically within differential diagnosis after seeing the morphology, in correlation with clinical and radiological details provided to the pathologist. Clinical and radiological details are mandatory in the interpretation of morphology of bone tumors and the selection of appropriate immunostains.
Conflict of Interest
The authors wish to express that they have no conflict of interest.
References
1. Netto GJ, Epstein JI. Theranostic and prognostic biomarkers: genomic applications in urological malignancies. Pathology 2010; 42(4):384-394.
2. Fanburg JC, Rosenberg AE, Weaver DL, Leslie KO, Mann KG, et al. Osteocalcin and osteonectin immunoreactivity in the diagnosis of osteosarcoma. Am J Clin Pathol. 1997; 108(4):464-473.
3. Fanburg-Smith JC, Bratthauer GL, Miettinen M. Osteocalcin and osteonectin immunoreactivity in extraskeletal osteosarcoma: a study of 28 cases. Hum Pathol. 1999; 30(1):32-38.
4. Fanburg-Smith JC, Auerbach A, Marwaha JS, Wang Z, Rushing EJ. Reappraisal of mesenchymal chondrosarcoma: novel morphologic observations of the hyaline cartilage and endochondral ossification and beta-catenin, Sox9, and osteocalcin immunostaining of 22 cases. Hum Pathol. 2010; 41(5):653-662.
5. Grifone TJ, Haupt HM, Podolski V, Brooks JJ. Immunohistochemical expression of estrogen receptors in chondrosarcomas and enchondromas. Int J Surg Pathol.
2008; 16(1):31-37.
6. Oien KA, Dennis JL. Diagnostic work-up of carcinoma of unknown primary: from immunohistochemistry to molecular profiling. Ann Oncol. 2012; 23 Suppl 10:271-277.
7. Hammas N, Laila C, Youssef AL, Hind el F, Harmouch T, et al. Can p63 serve as a biomarker for giant cell tumor of bone? A Moroccan experience. Diagn Pathol. 2012; 7:130.
8. Pang ZG, He XZ, Wu LY, Wei W, Liu XY, et al. Clinicopathologic and immunohistochemical study of 23 cases of mesenchymal chondrosarcoma. Zhonghua Bing Li Xue Za Zhi 2001; 40(6):368-372.
9. Rosai and Ackerman’s Surgical pathology, Tenth edition chapter 24.
10. Shakked RJ, Geller DS, Gorlick R, Dorfman HD. Mesenchymal chondrosarcoma: clinicopathologic study of 20 cases. Arch Pathol Lab Med. 2012; 136(1):61-75
11. Wehrli BM, Huang W, De Crombrugghe B, Ayala AG, Czerniak B. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum Pathol. 2003; 34(3):263-269.
12. Lee AF, Hayes MM, Lebrun D, Espinosa I, Nielsen GP, et al. FLI-1 distinguishes Ewing sarcoma from small cell osteosarcoma and mesenchymal chondrosarcoma. Appl Immunohistochem Mol Morphol. 2011; 19(3):233-238.
13. Tomlins SA, Palanisamy N, Brenner JC, Stall JN, Siddiqui J, et al. Usefulness of a monoclonal ERG/FLI1 antibody for immunohistochemical discrimination of Ewing family tumors. Am J Clin Pathol. 2013; 139(6):771-779.
14. Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006; 29(1):96-99.
15. Olivera P, Perez E, Ortega A, Terual R, Gomes C, et al. Estrogen receptor expression in giant cell tumors of the bone. Hum Pathol. 2002; 33(2):165-169.
16. D'cruze L, Dutta R, Rao S, R A, Varadarajan S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013; 7(7):1377-1382.