Full Text
Introduction
Brucellosis is an infection that affects mammals’ cells caused by a species from the genus Brucella, a gram-negative, immobile, facultative, intracellular, and microaerophilic coccobacillus. In humans, the pathogen species are Brucella melitensis, B. abortus, B. suis and B. canis [1-3]. Mexico is one of the countries with the highest incidence of human brucellosis in Latin America. Despite the programs implemented in the animal population and the progress achieved, it currently continues to be a public health problem. Its incidence has been reported of up to 3.7/100,000 inhabitants in women and up to 2.1/100,000 inhabitants in men (a possible explanation for this phenomenon is that women in Mexico tend to attend health centers more frequently than men, and more cases are detected in them, while in other countries, the highest incidence rates are found in males). Despite the fact that the disease has mortality in humans of less than 5%, the impact is mainly economic and social due to the high costs of its diagnosis, treatment and the disabilities caused [4]. We are presenting a case with confirmed brucellosis and the cardiovascular, cutaneous, gastrointestinal, hepatic, osteomuscular, renal, and hematological complications that came with it.
Case report
The patient is a 62-year-old man whose only relevant antecedent is the consumption of rural-elaborated cheeses. His symptomatology started with intermittent and irregular fever episodes (reaching up to 39.2°C/102.56°F), accompanied by profuse diaphoresis, asthenia, intermittent, generalized, abdominal pain level 6/10 of intensity, and, diarrhea (consistency Bristol type 7) up to 3 times a day. He went to a private physician, received treatment with amoxicillin-clavulanic acid for 7 days and improved partially.
2 months later, the fever and diaphoresis episodes returned with new purpuric lesions in pelvic members at distal zone up to both knees. The following days more symptoms appeared: mild exertional dyspnea, progressive esophageal dysphagia, low back pain, myalgia, and arthralgia in knees, hips and ankles. The patient asked for medical reassessment and the new tests reported antigen for Brucella abortus 1:320, typhi H 1:60 and paratyphi 1:80. Treatment started with gentamicin and doxycycline, only for 5 days.
The following month, the symptomatology began to worsen and dyspnea at rest appeared, so he went to urgent care. Upon admission, he suffered from hypoxemia at room air, which improved after applying supplementary oxygen. There were clinical findings related to decompensated heart failure (both in pulmonary examination and for bimalleolar peripheral edema); precordial auscultation with pericardial friction rub and mesosystolic murmur in aortic focus grade II/VI with irradiation to both carotids; somnolence, disorientation; dehydration; and, palpable purpura on both legs (Figure 1). He was hospitalized then.
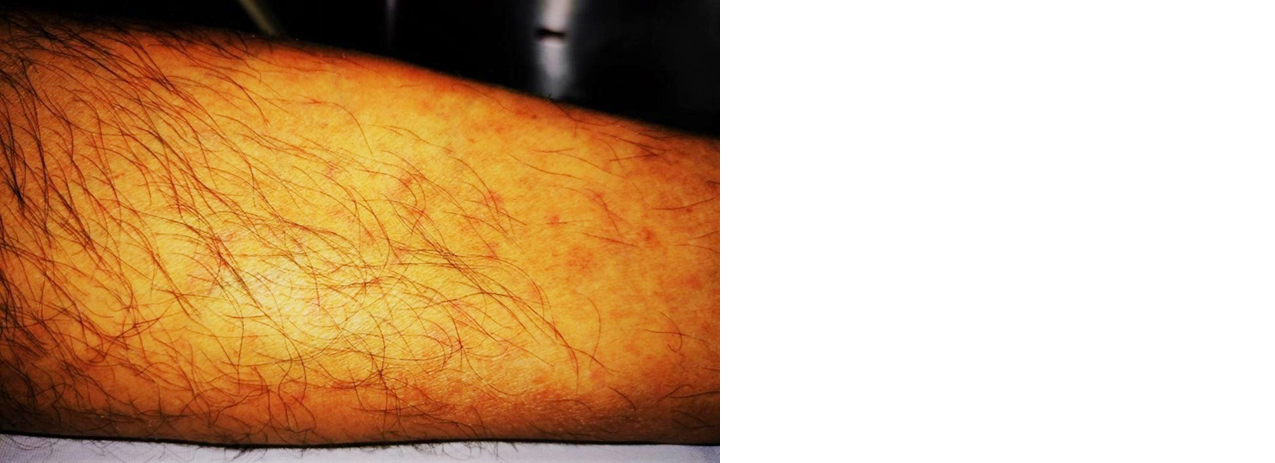
Figure 1: Purpuric lesions on both legs. His initial study protocol highlights: regenerative normocytic normochromic anemia (hemoglobin: 10.6 g/dL); leukocytosis (11,940/μl) due to neutrophilia; acute kidney injury (creatinine: 1.58 mg/dL, urea: 68.2 mg/dL); high ESR, PCR, ferritin (1552 ng/mL) and LDH (442 U/l, normal: 135-225 U/l); hypoalbuminemia (2.54 g/dL); cholestasis with total bilirubin of 2.08 due to direct hyperbilirubinemia, GGT: 306 U/l (normal: 8-65 U/l) and alkaline phosphatase: 169 U/l (normal: 40-129 U/l). The urinalysis did not detect urinary tract infections or visible alterations in the sediment. A chest x-ray showed cardiomegaly and central fluid overload.
The agglutination in the Rose Bengal test was positive, thus new confirmatory studies for brucellosis were requested: positive 2-mercaptoethanol with dilution 1:80, blood culture with development of Brucella melitensis, and a Coombs test, which resulted positive. Infectiology prescribed ciprofloxacin and tetracycline, since rifampicin, gentamicin and streptomycin were unavailable in our hospital. Also, on account of heart failure, the patient stayed in supplementary oxygen and continued taking furosemide.
In a bone marrow culture, there was no evidence of infection by B. mellitensis. When the patient developed neurological alterations, a cranial CT scan and a complete cerebrospinal fluid analysis were requested, but neither showed structural alterations or neuroinfection data; thus, the mental alterations were attributed to hypoactive delirium. Later, in a performed transthoracic echocardiography we can highlight the undiluted hypertrophied left ventricle; a diastolic dysfunction; a preserved systolic function (left ventricle ejection fraction of 60%); double aortic injury (stenosis, both mild) without vegetations nor abscesses; right ventricle with a slight dilatation in its basal diameter; and, a mild pericardial effusion without hemodynamic repercussions. About the low back pain mentioned by the patient, a spine and hip CT scan discarded visible bone lesions, injuries in liver parenchyma or dilatation of the intrahepatic or extrahepatic biliary tract, but confirmed the cardiomegaly and pericardial effusion observed in the previous echocardiogram (Figure 2).
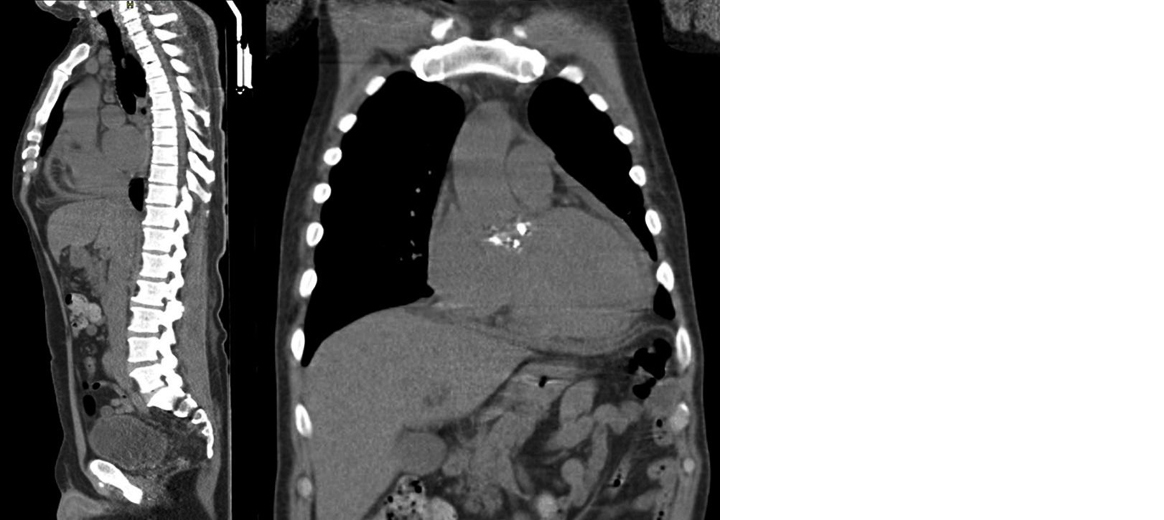
Figure 2: Cardiomegaly and pericardial effusion.
Because of the lower dysphagia, an upper endoscopy was performed and reported esophageal candidiasis (Kodsi I) and chronic erosive pangastropathy (Figure 3), so fluconazole was added to his treatment. Serology was all negative for human immunodeficiency virus, hepatitis, and TORCH.
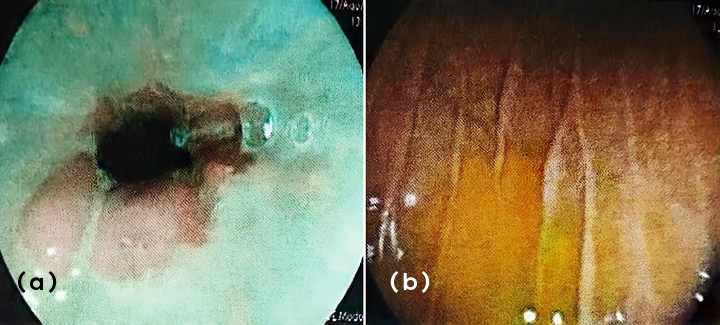
Figure 3a,b: Esophageal candidiasis and erosive pangastropathy.
During the following days of his hospitalization, the patient improved and his fever, diarrhea, abdominal and low back pain, arthralgia, dyspnea, peripheral edema, and other biochemical parameters decreased. Also, there was an important reduction in acute phase reactants, leukocytes, nitrogen compounds, bilirubins and other hepatic tests. He was discharged after 23 days of hospitalization and kept following up by external consultations. Yet, 4 months later the signs of cardiovascular deterioration returned, with symptoms like heart failure at rest, and he was again admitted to the cardiology unit at medical care level 3. In a transthoracic echocardiography an intracavitary mass was identified (Figure 4), located in the aortic valve, where also there was a reduction in the left ventricle ejection fraction. Surgical intervention was considered, but it was not possible to perform due to the fast worsening of his secondary hemodynamic condition that turned into unstable tachyarrhythmia. Despite the intense care provided for his heart failure, the patient passed away.
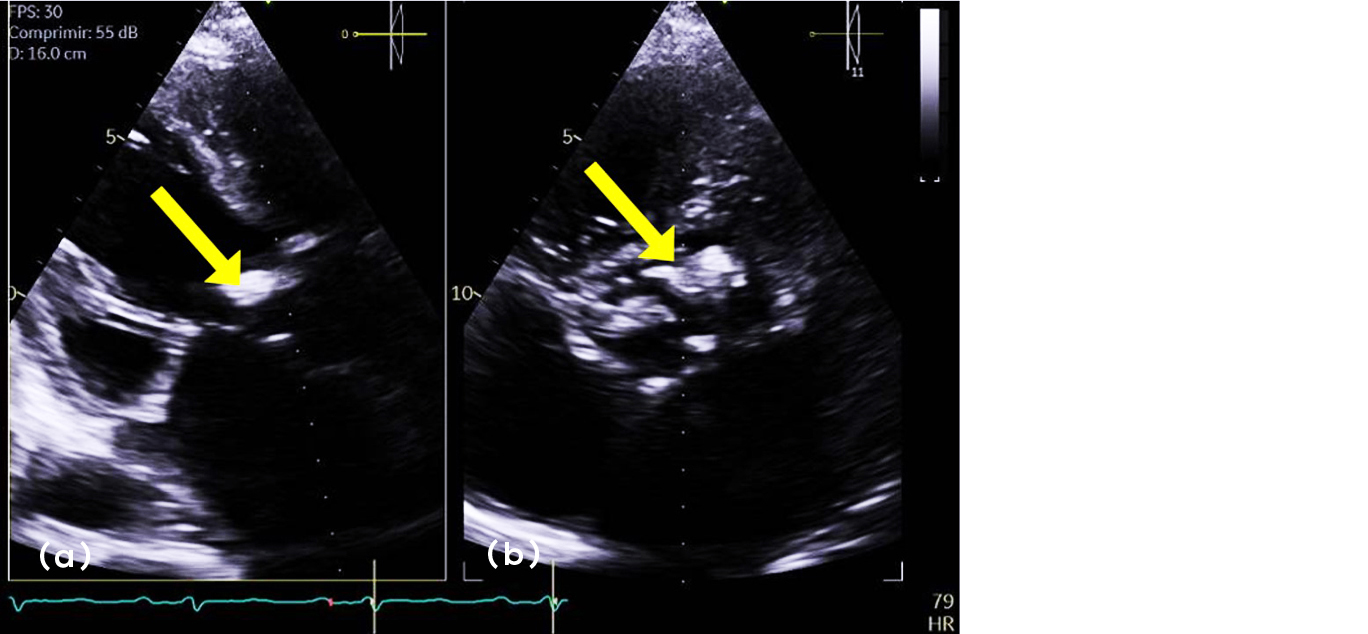
Figure 4a,b: Intracavitary mass in the aortic valve shown in the transthoracic echocardiography.
Discussion
Brucellosis, also known as undulant fever or Malta fever, is a zoonosis with great relevance yet in these days, enlisted in the illnesses caused by occupational exposure per the International Labour Organization. The main infection causes are through the means of ingesting unpasteurized milk and contacting infected animals [5, 6]. The seroprevalence in farmers corresponds to 18.1%, from which 13.3% had signs of recent infection. Residing in a high prevalence site confers an odds ratio of 2.2 (IC 95% 1.1-4.4) and is linked to prolonged time of exposure [7]. It has a higher incidence rate in bovines (11.28%), followed by goats (6.57%); which rises the human brucellosis incidence rate to 15% and 33% respectively [4]. According to Dermidal and Sen, it typically occurs in patients older than 50 years old (p >0.001) and it is often associated with males, yet this is not significant [8].
Brucellosis is considered an acute infection if 0-2 months have passed, subacute after 2-12 months and chronic after >12 months [9]. The illness is acute in half of the reported cases, with an incubation period of two to three weeks, while the clinical picture of the other half of cases is insidious due to unspecified symptoms and signs that develop in a period of weeks and months [5]. When acute, its characteristic symptomatology is fever (62% of cases), diaphoresis (48-68%), arthralgia (47-70%), myalgia (30%), low back pain (31-36%), neurological alterations (0.1-3.1%), and nausea (1.1-10%) [9, 10].
There are located forms of this disease that generally appear in 30% of patients (Table 1) [5]. From these, our clinical case showed a combination of cutaneous (palpable purpura, compatible with leukocytoclastic vasculitis), digestive (diarrheal syndrome and non-obstructive cholestasis), cardiovascular (possible myocarditis and pericardial effusion attributed to pericarditis, explaining the heart failure of the patient together with his valvulopathies), osteomuscular and hematological (anemia) manifestations, which were attributed to this infection and improved with treatment. Regarding his acute kidney injury, the dissociation between nitrogen compounds and non-active urine sediment suggested a prerenal origin (from a multifactorial cause: sepsis, decompensated heart failure, dehydration), also associated to an inherent cause (glomerulonephritis, interstitial nephritis). Neurobrucellosis was ruled out as a direct cause of the patient’s mental alterations.
Table 1: Manifestations located in brucellosis [5].
|
Complications
|
Manifestations
|
|
Osteoarticular
|
Arthritis
|
|
Spondylitis
|
|
Sacroiliitis
|
|
Bursitis
|
|
Osteomyelitis
|
|
Synovitis
|
|
Genitourinary
|
Glomerulonephritis
|
|
Interstitial nephritis
|
|
Orchitis
|
|
Epididymitis
|
|
Prostatitis
|
|
Cystitis
|
|
Neurological
|
Meningitis
|
|
Encephalitis
|
|
Myelitis and neuritis
|
|
Brain abscess
|
|
Depression and psychosis
|
|
Cardiovascular
|
Endocarditis
|
|
Myocarditis
|
|
Pericarditis
|
|
Digestive
|
Hepatic abscess
|
|
Granulomatous and diffuse hepatitis
|
|
Cholecystitis
|
|
Cutaneous
|
Erythema nodosum
|
|
Leukocytoclastic vasculitis
|
|
Exanthem (macular, papular, etc.)
|
|
Pulmonary
|
Bronchopneumonia
|
|
Interstitial pneumopathy
|
|
Empyema
|
|
Hilar adenopathy
|
|
Cavitation
|
|
Hematological
|
Intravascular coagulation
|
|
Anemia
|
|
Leukopenia
|
|
Thrombocytopenia
|
|
Pancytopenia
|
|
Others
|
Uveitis
|
|
Thyroiditis
|
In order to diagnose brucellosis, the gold standard is a positive culture from bone marrow, peripheral blood, or other tissues; although most of the literature reports that peripheral blood is suboptimal [11] with a detection only of 45.6%, lesser than the 82.5% in bone marrow culture (p <0.001) [12]. However, Sehabi and cols. described a greater sensitivity in hemoculture (44.4%) than in myeloculture (27.7%) for the detection of Brucella melitensis [13].
Serology is useful to classify the phase or state of infection. In this case, based on availability, 2-Mercaptoethanol was requested, which allows to eliminate the IgM bias by inactivating the S-S bonds of its pentameric structure and even the IgA dimer, keeping IgG positive. This way we can get a positive after the first two weeks [11] and have a diagnosis range of 1:40 to 1:80 in low incidence zones and ≥1:80 in endemic zones or individuals with repeated exposition of Brucella [14], as it was the case of our patient. Other diagnostic methods are: tube agglutination (SAT), complement fixation, Coombs, and polymerase chain reaction in blood [5].
In a cross-sectional study of brucellosis diagnostic confirmation in Puebla, México, Arciga and peers emphasize the importance of serology for the diagnosis of this disease due the low specificity of the symptoms. Only 50% of patients with suspected brucellosis test positive [15].
The treatment for acute brucellosis is administered according to three schemes by the World Health Organization (Table 2) [5]. Generally, the treatment for patients without endocarditis, spondylitis and neurobrucellosis takes about 2-3 up to 6 weeks when including daily doxycycline 200mg plus an aminoglycoside (streptomycin or gentamicin) or rifampicin respectively, being the latter a secondary option due to the risk of mycobacterial resistance and higher failure or relapse rate [16].
Table 2: Treatment schemes suggested by the World Health Organization [5].
|
Type of scheme
|
Suggested drugs, dosage, and treatment duration
|
Considerations
|
|
Scheme A
|
Tetracycline, 500 mg oral route, every 6 hours for 6 weeks.
|
Take 2 hours before meals with 500 ml of water.
|
|
Streptomycin, 1 g intramuscular every 24 hours for 21 days.
As an alternative, gentamicin 5 mg/kg/day for 14 days.
|
Use with caution in patients with nephropathy and hearing or balance impairment.
|
|
Scheme B
|
Rifampicin, 300 mg oral route, every 8 hours for 21 days.
Children: 20 mg/kg/day.
|
Decreased effect of oral anticoagulants, hypoglycemic agents, and oral contraceptives.
|
|
Trimethoprim/sulfamethoxazole, 320/1600 mg/day oral route, for 21 days.
Children: 8/40 mg/kg/day.
|
Folic acid levels monitoring.
|
|
Scheme C
|
Doxycycline, 200 mg oral route, every 24 hours for 6 weeks.
|
Take 2 hours before meals with 500 ml of water and do not ingest with dairy products or antacids.
|
|
Rifampicin, 600 to 900 mg oral route, every 24 hours for 6 weeks.
|
As stated above.
|
Due to the unavailability of first-line drugs, we considered other kinds of drugs for our patient. In literature, there are reports of treatment schemes with quinolones and sulfonamides. According to a comparative essay with ciprofloxacin-rifampicin, ciprofloxacin-doxycycline and doxycycline-rifampicin, there was no significant difference in response between the three schemes (p= 0.09) [17]. Another alternative included the use of levofloxacin and doxycycline for 90 days in patients without systemic involvement, which has demonstrated culture negativization, although up to 44.9% of patients keep being seropositive and symptoms persist in 1.9% of patients [18]. For this reason, treatment with quinolone and tetracycline was indicated and the patient had a favorable response to the scheme until his relapse 4 months after his discharge presenting main myocardial function deterioration, subsequently passing away despite the management in the cardiology unit. We have to remember that some attributed manifestations in a brucellosis infection may include tachyarrhythmia, myocarditis, endocarditis, pericarditis and pseudotumors [19], which can cause fatal outcomes.
We want to emphasize the conditions that contribute to the transmission. In Mexico, there is a high production of beef cattle without disease limitation strategies nor sanitary regulation of dairy and meat products [20].
We must exhaust all the diagnostic resources to confirm this entity in those with suspected infection, since it will allow the establishment of an appropriate treatment and prevent the risk of complications and even death. In countries with high prevalence, the dissemination of preventive measures is crucial.
Finally, despite being an old disease, there are still many aspects to be discovered. For this reason, based on the evidence presented, we propose the following potential areas of research on brucellosis: (1) To better understand the mechanisms of pathogenicity of Brucella, (2) Identify new strains of this infectious agent throughout the world, (3) Improve diagnostic tools for this disease, (4) Define new alternative treatment schemes, (5) Create better strategies to prevent the spread of this infection (mainly in high prevalence countries), (6) Develop an effective and economically accessible human vaccine for all countries.
Conclusions
It is necessary to be highly suspicious of this infection, since its non-specific nature can be challenging for the physician. This is due to the large number of clinical presentations in various organs that brucellosis can present (such as those mentioned in this clinical case, which had cardiovascular, skin, gastrointestinal, hepatic, osteomuscular, renal, and hematological complications) and that can be simultaneous or summative.
Acknowledgement
To Dr. Esther Asunción César Arce, infectologist, for her support in this clinical case during the patient’s hospitalization in our unit.
Conflicts of interest
The authors declare to not have any conflicts of interest.
References
[1] Eisenberg T, Hamann HP, Kaim U, Schlez K, Seeger H, et al. Isolation of potentially novel Brucella spp from frogs. Appl Environ Microbiol. 2012; 78:3753–5755.
[2] Ficht T. Brucella taxonomy and evolution. Future Microbiol. 2010; 5:859–866.
[3] Gul H, Erdem H. Brucelosis (especies de Brucella). En: Bennett J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Barcelona: Elsevier, 2020; pp.2753–2758.
[4] Méndez M, Rodríguez E, Sánchez L. Brucelosis, una zoonosis presente en la población: estudio de series de tiempo en México. Salud Pública de México. 2015; 57:519–527.
[5] Vega-López CA, Ariza-Andraca R, Rodríguez Weber FL. Brucelosis: una infección vigente. Acta Médica Grupo Ángeles. 2008; 6:158–165.
[6] International Labour Organization. ilo. 2010. Accessed on 25 September 2021 from: https://www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---safework/documents/publication/wcms_125137.pdf.
[7] Cervera-Hernández M, Ordaz-Vázquez A, Torres-Gonzalez P, Chávez-Mazaeri B, Soberanis-Ramos O, et al. Seroprevalence of brucellosis among dairy farm workers in Mexico. Salud Pública Méx. 2016; 58:366–370.
[8] Demirdal T, Sen P. Risk factors for focal involvement in brucellosis. Diagnostic Microbiology & Infectious Disease. 2020; 97:1–5.
[9] Hasanjani RMR, Ebrahimpour S, Moulana Z. Different clinical presentations of Brucellosis. Jundishapur J Microbiol. 2016; 9:e33765.
[10] Kayaaslan B, Bastug A, Aydin E, Akinci E, But A, et al. A long-term survey of brucellosis: Is there any marker to predict the complicated cases? Infectious Diseases. 2016; 48:1–7.
[11] Yagupsky P, Morata P, Colmenero JD. Laboratory Diagnosis of Human Brucellosis. Clin Microbiol Rev. 2019; 33:e00073–19.
[12] Mantur B, Mulimani M, Bidari L, Akki AS, Tikare NV. Bacteremia is as unpredictable as clinical manifestations in human brucellosis. Int J Infect Dis. 2008; 12:303–307.
[13] Shehabi A, Shakir K, El-Khateeb M, Qubain H, Fararjeh N, et al. Diagnosis and treatment of 106 cases of human brucellosis. J Infect. 1990; 20:5–10.
[14] Avijgan M, Rostamnezhad M, Jahanbani-Ardakani H. Clinical and serological approach to patients with brucellosis: A common diagnostic dilemma and a worldwide perspective. Microbial Pathogenesis. 2019; 129:125–130.
[15] Arciga-Vázquez G, Santos-López G, Castañeda-Roldan E, Cedillo-Ramírez ML, Cano-Vázquez EN, et al. Estudio de casos confirmados de brucelosis humana en Puebla, México. Rev Chilena Infectol. 2021; 38:281–289.
[16] Bosilkovski M, Keramat F, Arapovic J. The current therapeutical strategies in human brucellosis, Infection. 2021; 49:823–832.
[17] Keramat F, Ranjbar M, Mamani M, Hashemi SH, Zeraati F. A comparative trial of three therapeutic regimens: ciprofloxacin-rifampin,ciprofloxacin-doxycycline and doxycycline-rifampin in the treatment of brucellosis. Tropical doctor. 2009; 39:207–210.
[18] Wang H, Liu H, Zhang Q, Lu X, Li D, et al. Natural History of and Dynamic Changes in Clinical Manifestation, Serology, and Treatment of Brucellosis, China, Emerg Infect Dis. 2022; 28:1460–1465.
[19] Kaczmarek K, Wojnicz R, Ptaszynski P, Wranicz JK, Cygankiewicz I. Systemic Brucellosis with Arrhythmogenic Cardiac Inflammatory Pseudotumor, Am J Case Rep. 2022; 23:e935259.
[20] Lozano E, Austreberta-Hazar D, Nareh J. Brucelosis bovina y humana en el sur de México: Una zoonosis desatendida. Rev Chilena Infectol. 2022; 39:157–165.