Orginal Research
2023
June
Volume : 11
Issue : 2
The impact of COVID-19 on neonatal hearing in a tertiary care hospital
Thomas N, Pragathi BS, Kanimozhi KS, Seema GB, Blesson CS, Chaithra KC
Pdf Page Numbers :- 76-80
Nitha Thomas1,*, Pragathi BS1, Sakthi Kanimozhi K1, Seema GB1, Chinchu Sara Blesson1 and Chaithra KC1
1Department of ENT-HNS, Adichunchanagiri Institute of Medical Sciences, B.G.Nagara, Mandya District, Karnataka State 571448, India
*Corresponding author: Dr. Nitha Thomas, Assistant Professor (MBBS, DLO, MS, DNB), Department of ENT-HNS, Adichunchanagiri Institute of Medical Sciences, B.G.Nagara, Mandya District, Karnataka State 571448, India. Email: drnitha@bgsaims.edu.in
Received 16 December 2022; Revised 1 March 2023; Accepted 11 March 2023; Published 20 March 2023
Citation: Thomas N, Pragathi BS, Kanimozhi KS, Seema GB, Blesson CS, Chaithra KC. The impact of COVID-19 on neonatal hearing in a tertiary care hospital. J Med Sci Res. 2023; 11(2):76-80. DOI: http://dx.doi.org/10.17727/JMSR.2023/11-15
Copyright: © 2023 Thomas N et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Coronavirus disease 2019 (COVID-19) outbreak in China has had far reaching repercussions on mankind. Its effects on pregnant women and neonates have been intricate. The aim of the study was to assess the impact of gestational COVID-19 on neonatal hearing with the objectives of determining the factors affecting hearing and to estimate the prevalence of hearing loss in neonates whose mothers had gestational COVID-19.
Materials and methods: The hospital-based cross-sectional study included 60 neonates whose mothers had gestational COVID-19 infection and 60 neonates of healthy mothers as controls in a rural tertiary care hospital for a duration of 1 year from September 2020 to August 2021. The study and control groups were compared in terms of continuous and non continuous variables. Maternal age, birth week and birth weight were categorized as continuous variables. Trimester of RT-PCR positivity, parity, mode of delivery, gender and results of transient evoked otoacoustic emissions (TEOAE) were categorized as non continuous variables. All neonates were screened with TEOAE within the first 5 days of birth.
Results: The demographic and clinical characteristics of the study and control groups on comparison did not reveal any statistically significant differences. All neonates passed the screening test in the first attempt.
Conclusion: Otologic manifestations in COVID-19 has been diverse and is a cause of concern. Neonatal hearing loss was not observed in the study. A better understanding of this entity calls for further research as early detection can help to mitigate the aftermath of the infection if any.
Keywords: COVID-19; neonates; hearing loss; pregnancy; TEOAE; congenital infections; vertical transmission
Full Text
Introduction
In March 2020, WHO declared COVID-19 as a global pandemic due to its profound and devastating ripple effects on humanity. The myriad presentations including neurological manifestations of the disease varies from subtle to overt [1]. The ramifications of vertical transmission of infection on infant hearing is a cause for concern. Extrapolating from TORCH (toxoplasmosis, others (syphilis, hepatitis B), rubella, cytomegalovirus, herpes simplex) infections with hearing loss as a common sequelae, the potential for neurological damage in the inner ear by coronavirus infection is highly compelling [2, 3].
The study aimed to assess the impact of gestational COVID-19 on hearing in neonates with the objectives of determining the factors affecting hearing and to estimate the prevalence of hearing loss in neonates whose mothers had gestational COVID-19 using transient evoked otoacoustic emissions (TEOAE) as a screening test.
Materials and methods
The hospital-based cross-sectional study at Adichunchanagiri Institute of Medical Sciences was conducted for duration of 1 year from September 2020 to August 2021 after obtaining approval from the ethics committee (AIMS/IEC/1145/2020). It included 60 neonates whose mothers had gestational COVID-19 infection as subjects and 60 neonates of healthy mothers as controls. Written informed consent was obtained prior to the study which was performed in consonance with the declaration of Helsinki.
Pregnant women positive for COVID-19 with reverse transcription polymerase chain reaction (RT-PCR) test, maternal age of 20-40 years and neonates whose mothers tested positive for COVID-19 during gestation were included in the study.
Premature neonates (< 37 weeks of pregnancy), neonates with auricular and external auditory canal anomalies, neonates with hypoxia, neonatal jaundice necessitating exchange transfusions, COVID-19 positive pregnant women with TORCH infections & comorbidities, severe COVID-19 infection requiring intensive care and neonates with a high risk for hearing loss (Consanguineal) were excluded from the study.
The study and control groups were compared in terms of the following variables. Maternal age, birth week and birth weight were categorized as continuous variables. Trimester of RT-PCR positivity, parity, mode of delivery, gender and results of TEOAE were categorized as non continuous variables.
120 neonates were evaluated binaurally between 1-5 days of birth with TEOAE in a sound proof room, using Echolab TEOAE instrument. The recording was done with a non-linear click sequence stimulus having a click rate of around 60 Hz. The signal bandwidth ranged from 1000 Hz to 5000 Hz with an optimal sound pressure of 60-65 dB. The outcome of the test was interpreted as “Pass” or “Refer”.
None of the mothers were positive for the infection 14 days prior to the delivery, hence the precautionary RT-PCR test in neonates before the audiological assessment was negative. Also, the pregnant women were unvaccinated as they were excluded from vaccination drives in the first wave which coincided with the study period.
Statistical analysis
The analysis was performed using Statistical Package for Social Sciences (SPSS) version 21.0. Distributions of parameters for normality were analyzed by the Kolmogorov-Smirnov test and the Shapiro-Wilks test. None of the continuous variables followed the normal distribution. The Mann-Whitney U test was used to compare differences for each of the continuous variables. Mean, standard deviation and median (minimum to maximum) were used to describe the variables. Pearson’s Chi-square test was used for non continuous variables. A p value < 0.05 was considered significant.
Results
In the COVID-19 positive group, 13.3% and 6.7% were infected in the 1st and 2nd trimesters and 80% were infected in the 3rd trimester (Figure 1).
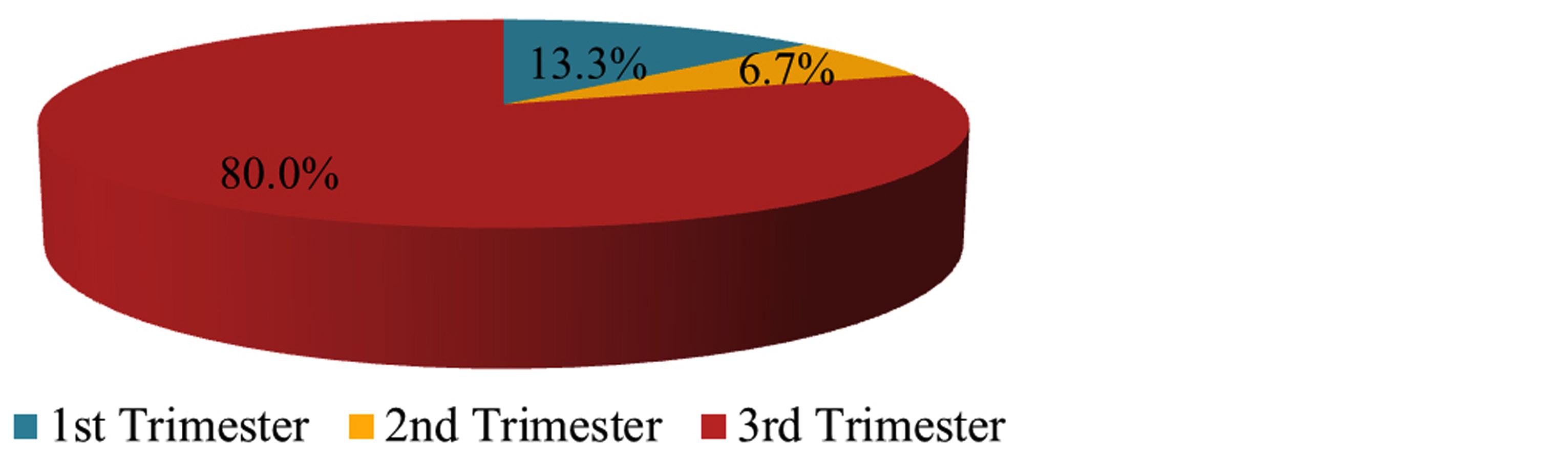
Figure 1: Trimester of infection (1st, 2nd, 3rd).
86.7% of mothers were in the 20-34 years age group and 13.3% were 35 years or more. 40% were primiparous and 60% were multiparous. 66.7% had a vaginal delivery (VD) and 33.3% underwent a caesarean section (CS). 46.7% were males and 53.3% were females.
In the healthy control group, 88.3% of mothers were in the 20-34 years age group and 11.7% were 35 years or more. 43.3% were primiparous and 56.7% were multiparous. 60% had a vaginal delivery and 40% underwent a caesarean section. 55% were males and 45% were females.
The demographic and clinical characteristics of pregnant women with COVID-19 infection and neonates along with the healthy control group on comparison did not reveal any statistically significant differences in terms of maternal age (p = 0.794), birth week (p = 0.797), birth weight (p = 0.282), babies’ gender (p = 0.361), parity (p = 0.711) and mode of delivery (p = 0.449).
All neonates passed the TEOAE test in the first screening. The outcome of the test was inferred as pass if a response was present in any of the 3 frequencies within the bandwidth. 12, 30 and 18 neonates had a response in 3,4 and 5 frequencies in the COVID group.11, 35 and 14 neonates had a response in 3,4 and 5 frequencies in the control group. Statistically significant difference was not seen between the groups (p = 0.629). Neonatal hearing loss was not observed in the study (Table 1).
Table 1: Demographic and clinical characteristics with hearing screening results of the COVID-19 and healthy control groups.
|
Characteristics
|
COVID (n = 60)
|
Control (n = 60)
|
P value
|
|
Age (Years)
|
|
Mean ± SD
|
26.08 ± 4.67
|
26.03 ± 4.89
|
0.794*
|
|
Median (Min, Max)
|
25 (20 - 36)
|
25 (20 - 37)
|
|
|
Birth week
|
|
Mean ± SD
|
38.4 ± 1.04
|
38.35 ± 1.02
|
0.797*
|
|
Median (Min, Max)
|
39 (37 - 40)
|
38 (36 - 40)
|
|
|
Birth weight (Kg)
|
|
Mean ± SD
|
2.97 ± 0.36
|
3.04 ± 0.38
|
0.282*
|
|
Median (Min, Max)
|
2.9 (2.3 - 3.75)
|
3 (2.4 - 3.8)
|
|
|
Gender
|
|
Male
|
28 (46.67%)
|
33 (55%)
|
0.361**
|
|
Female
|
32 (53.33%)
|
27 (45%)
|
|
|
Parity
|
|
Primiparous
|
24 (40%)
|
26 (43.33%)
|
0.711**
|
|
Multiparous
|
36 (60%)
|
34 (56.67%)
|
|
|
Type of delivery
|
|
VD
|
40 (66.67%)
|
36 (60%)
|
0.449**
|
|
CS
|
20 (33.33%)
|
24 (40%)
|
|
|
TEOAE frequency
|
|
3 kHz
|
12 (20%)
|
11 (18.33%)
|
0.629**
|
|
4 kHz
|
30 (50%)
|
35 (58.33%)
|
|
|
5 kHz
|
18 (30%)
|
14 (23.33%)
|
|
Note: *Mann-Whitney U test; **Pearson’s Chi-Square test; p<0.05 is considered significant.
Discussion
Novel coronavirus disease, named after the Latin word “crown” is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This respiratory tract infection is exceedingly contagious and is known to engender severe complications involving other systems also. They are neurotropic viruses and can migrate to the cranial nervous system through the motor proteins, kinesins and dynein [4, 5]. Angiotensin-converting enzyme 2 (ACE 2) receptors, the targets for the virus are expressed in many neurons and non-neuron cells, which when involved leads to a wide range of neurological sequelae [6].
Inner ear develops early through carnegie stages 14 to 16 and can have immune mediated damage. COVID-19 can cause direct or indirect ear damage, the molecular mechanisms of which are inadequately explored [7, 8]. Viruses targeting the ACE 2 receptors in the temporal lobe, the midpoint of hearing and its vicinity induces excessive cytokine secretion causing hearing impairments by oxidative destruction in the inner ear hair cells and organ of Corti [9].
Immune suppression in pregnancy makes pregnant women more susceptible to infections. Vertical maternal-fetal transmission in utero, intrapartum and via breast feeding is a major concern of the infection. Although it is plausible from a pathophysiological perspective, the actual in-utero infections are estimated at 2% [10].
Advanced maternal age including elderly primiparous and multiparous women are known to have a higher risk of complications and adverse birth outcomes but COVID-19 infection and disease severity is not associated with increased parity among pregnant women as correlated in this study [11]. Variable post COVID pregnancy outcomes with maternal and fetal morbidities have also been reported. Premature rupture of membranes, preterm delivery, low birth weight, fetal growth restriction, fetal distress, perinatal morbidity are a few of them [12-14].
The trimester in which the mother contracted the infection has a direct bearing on the fetal outcome. The 1st trimester (<13 weeks) is the period of organogenesis and is the most sensitive for the development of the ear. Fever during this trimester is potentially dangerous leading to death of dividing neuroblasts, disruption of cell migration and vascular damage making it vulnerable to infections and ototoxic insults [2].The high levels of TNF-alpha levels in the maternal blood can have a toxic effect on embryos in the early embryo period [15].
The 2nd trimester (13-28 weeks) with over all decreased immunity can harm the inner ear through host- immune response [16]. Pro inflammatory state and cytokine storm in the 1st and 3rd trimesters (increased IL-2, IL-7, IL-10, GCSF, Gamma MCP-1, TNF-alpha) in the mother can negatively impact fetal brain development [17]. Immunological changes like decreased number of T cells, decreased cell mediated cytotoxicity and decreased response to proliferation of lymphocytes is more prevalent in 2nd and 3rd trimesters [18]. Decreased lung volumes and respiratory capacity caused by increasing size of uterus along with co-morbidities may cause rapid clinical deterioration with COVID-19 during pregnancy. It is more pronounced in the 3rd trimester (>28 weeks) and may increase the risk of adverse pregnancy outcomes [19, 20].
Vertical transmission is possible during normal delivery but there is no clear evidence that caesarean delivery prevents transmission or if vaginal delivery could increase the transmission [20]. Expression of ACE 2 receptors is deficient in all kinds of early maternal fetal interface cells more so at term and could explain the reason of intrauterine transmission being low [21]. In contrast, presence of the virus in the placenta, amnion and blood of newborns along with IgM antibodies which do not cross placenta is a sign of intrauterine transmission [22, 23].
A proclivity of SARS-CoV-2 for the auditory system causing auditory and vestibular symptoms have been documented [24-26]. Studies related to the implications of the maternal infection on neonatal hearing is deficient and with diverging results. Majority of the studies concluded that COVID-19 during pregnancy may not be a risk factor for hearing loss in neonates. On the contrary, insufficiency of medial olivocochlear efferent system and delayed neuromaturation affecting hearing in neonates born to mothers with gestational COVID-19 infection have also been reported [2, 27].
The organ of Corti is the sensory organ of hearing and has inner and outer sensory hair cells. Otoacoustic emissions (OAE) are sounds produced by the hair cells in response to an auditory stimulation. It is a simple and objective indicator of subtle cochlear damage. It is portable and can be recorded in the ear canal. It is generated by a reflection of the travelling wave in the organ of Corti as described by Kemp in 1978 [2, 28, 29].
TEOAE is a non invasive screening tool for the detection of hearing loss in infants and is more than 90% sensitive (80%-96.5%), and specific (90.60%-92.85%). It records the mechanical capacity as well as the mobility of the outer hair cells in response to a transient click [29, 30].
Limitations
The study has its limitations. The number of cases in the first and second trimesters having the infection was less. As the study was carried out in a single center with a small sample size, the results obtained by it cannot be deduced to a larger population. Also, with a possibility of an asymptomatic infection, false negatives in the control group cannot be ruled out.
Conclusion
Otologic manifestations in COVID-19 has been diverse and is a cause of concern. In this study, vertical transmission of SARS-CoV-2 from mothers to newborns causing neonatal hearing loss was not witnessed. A better understanding of this entity calls for further research as early detection can help with rehabilitation and alleviate the consequence of the infection if any. In conclusion, whether COVID-19 should be included as a risk factor for congenital hearing loss is contentious given the transitional scenario of the virus and the infection.
Acknowledgement
The parents who, braving the pandemic, consented to the study are gratefully acknowledged.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Oskovi-Kaplan ZA, Ozgu-Erdinc AS, Buyuk GN, Sert-Dinc UY, Ali-Algan C, et al. Newborn hearing screening results of infants born to mothers who had COVID-19 disease during pregnancy: A retrospective cohort study. Ear Hear. 2022; 43:41–44.
[2] Kosmidou P, Karamatzanis I, Tzifas S, Vervenioti A, Gkentzi D, et al. hearing outcomes of infants born to mothers with active COVID-19 infection. Cureus. 2022; 14:e25571.
[3] Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MA, et al. Congenital hearing loss. Nat Rev Dis Primers. 2017; 3:16094.
[4] Dey M, Singh S, Tiwari R, Nair VG, Arora D, et al. Pregnancy outcome in first 50 SARS-COV-2 positive patients at our center. Gynecol Obstet Reprod Med. 2021; 27:11–16.
[5] Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020; 87:18–22.
[6] Ahmed MU, Hanif M, Ali MJ, Haider MA, Kherani D, et al. Neurological manifestations of COVID-19 (SARS-CoV-2): A Review. Front Neurol. 2020;11:518.
[7] Lavigne-Rebillard M, Dechesne C, Pujol R, Sans A, Escudero P. Development of the internal ear during the 1st trimester of pregnancy. Differentiation of the sensory cells and formation of the 1st synapses (Article in French). Ann Otolaryngol Chir Cervicofac. 1985; 102:493–498.
[8] Karimi-Boroujeni M, Zahedi-Amiri A, Coombs KM. Embryonic origins of virus-induced hearing loss: overview of molecular etiology. Viruses. 2021; 13:71.
[9] Krasowska S. Neurotropism of SARS-CoV-2 in idiopathic hearing disorders. J Pre-Clin Clin Res. 2021; 15:100–103.
[10] Allotey J, Chatterjee S, Kew T, Gaetano A, Stallings E, et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022; 376:e067696.
[11] Marsden KA, Ten Eyck PP, Maxwell TN. COVID-19 infection and disease severity not associated with increased parity among pregnant women. J Med Clin Res Rev. 2021; 5:1–4.
[12] Veeranna SA, Youngblood PL, Bradshaw L, Marx CG. COVID-19 during pregnancy and its impact on the developing auditory system. Am J Otolaryngol. 2022; 43:103484.
[13] Sousa ÁFL, Carvalho HEF, Oliveira LB, Schneider G, Camargo ELS, et al. Effects of COVID-19 infection during pregnancy and neonatal prognosis: what is the evidence? Int J Environ Res Public Health. 2020; 17:4176.
[14] Wastnedge EA, Reynolds RM, Van Boeckel SR, Stock SJ, Denison FC, et al. Pregnancy and COVID-19. Physiol Rev. 2021; 101:303–318.
[15] Celik T, Simsek A, Koca CF, Aydin S, Yasar S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am J Otolaryngol. 2021; 42:102982.
[16] Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021; 224:3553.
[17] Figueiredo CP, Fontes-Dantas FL, da Poian AT, Clarke JR. SARS-CoV-2-associated cytokine storm during pregnancy as a possible risk factor for neuropsychiatric disorder development in post-pandemic infants. Neuropharmacology. 2021; 201:10884.
[18] Brito V, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med. 2011; 32:121–132.
[19] Turan O, Hakim A, Dashraath P, Jeslyn WJL, Wright A, et al. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS-CoV-2 infection among hospitalized pregnant women: A systematic review. Int J Gynaecol Obstet. 2020; 151:7–16.
[20] Cai J, Tang M, Gao Y, Zhang H, Yang Y, et al. Cesarean section or vaginal delivery to prevent possible vertical transmission from a pregnant mother confirmed with COVID-19 to a neonate: A systematic review. Front Med (Lausanne). 2021; 8:634949.
[21] Qing-Liang Z, Tao D, Li-Ping J. Single-cell RNA expression profiling of ACE2 and AXL in the human maternal-fetal interface. Reproduct Dev Med. 2020; 4:7–10.
[22] Mostafa BE, Mostafa A, Fiky LME, Omara A, Teaima A. Maternal COVID-19 and neonatal hearing loss: a multicentric survey. Eur Arch Otorhinolaryngol. 2022; 279:3435–3438.
[23] Konstantinidou AE, Skaltsounis P, Eleftheriades M, Antsaklis P, Charitou A, et al. Hellenic Society of Perinatal Medicine. Pharyngeal sampling for PCR-testing in the investigation of SARS-COV-2 vertical transmission in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2021; 260:18–21.
[24] Dharmarajan S, Bharathi MB, Sivapuram K, Prakash BG, Madhan S, et al. Hearing loss-a camouflaged manifestation of COVID 19 infection. Indian J Otolaryngol Head Neck Surg. 2021; 73:494–498.
[25] Kaliyappan K, Chen YC, Muthaiah VPK. Vestibular cochlear manifestations in COVID-19 cases. Front Neurol. 2022; 13:850337.
[26] De Luca P, Di Stadio A, Colacurcio V, Marra P, Scarpa A, et al. Long COVID, audiovestibular symptoms and persistent chemosensory dysfunction: A systematic review of the current evidence. Acta Otorhinolaryngol Ital. 2022; 42:S87–S93.
[27] Gallus R, Melis A, De Luca LM, Rizzo D, Palmas S, et al. The impact of COVID-19 on universal newborn hearing screening. Ear Hear. 2022; 43:1917–1919.
[28] Zimatore G, Cavagnaro M, Skarzynski PH, Fetoni AR, Hatzopoulos S. Detection of age-related hearing losses (ARHL) via transient-evoked otoacoustic emissions. Clin Interv Aging. 2020; 15:927–935.
[29] Kemp DT. Otoacoustic emissions, their origin in cochlear function and use. Br Med Bull. 2002; 63:223–241.
[30] Kosmidou P, Tzifas S, Lygeros S, Danielides G, Nikolopoulos T, et al. Newborn hearing screening: analysing the effectiveness of early detection of neonatal hearing loss in a hospital in Greece. Cureus. 2021; 13:e19807.