Orginal Research
2023
September
Volume : 11
Issue : 3
Clinicopathological spectrum of renal biopsies in children – A single center experience from Eastern India
Kanjilal S, Hui P, Roy SM, Das MK, Basu S, Sinha MK
Pdf Page Numbers :- 176-180
Shruti Kanjilal1, Pallabi Hui1, Sarbani Misra Roy2,*, Mrinal Kanti Das1, Suprit Basu1,3 and Malay Kumal Sinha1
1Department of Pediatrics, IPGME&R, Kolkata, West Bengal 700020, India
2Department of Pediatrics, Malda Medical College and Hospital, Malda, West Bengal 732101, India
3Department of Pediatric Rheumatology, PGIMER Chandigarh, Chandigarh 160012, India
*Corresponding author: Sarbani Misra Roy. Associate Professor, Department of Pediatrics, Malda Medical College and Hospital, Malda-732101. West Bengal, India. Email: misra.sarbani@gmail.com
Received 3 April 2023; Revised 23 May 2023; Accepted 1 June 2023; Published 14 June 2023
Citation: Kanjilal S, Hui P, Roy SM, Das MK, Basu S, Sinha MK. Clinicopathological spectrum of renal biopsies in children – A single center experience from Eastern India. J Med Sci Res. 2023; 11(3):176-180. DOI: http://dx.doi.org/10.17727/JMSR.2023/11-33
Copyright: © 2023 Kanjilal S et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Renal diseases are common in childhood and they often present with diagnostic challenges. Renal biopsy is of major importance in diagnosing many renal diseases in children. The aim of this study was to evaluate the clinicopathological aspect of renal diseases underwent biopsy in children in a tertiary care teaching institute in Eastern India.
Materials and methods: This cross sectional study was conducted on children (≤12 year) admitted with difficult to treat renal disorders in Pediatric ward and thorough evaluations confirmed the requirement of renal biopsy. Sixty one (61) children satisfying the inclusion and exclusion criteria were enrolled in our study. Period of study was from February 2021 to July 2022.
Results: In this study, males were 31 and females were 30 in numbers. The mean age was 6.77± 3.42 years. The indications for renal biopsy were steroid resistant nephrotic syndrome (SRNS) (49.18%), systemic lupus erythematosus (SLE) (29.51%), acute glomerulonephritis (AGN) (16.39%), and others. The major bulks of histopathological findings revealed focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis (MPGN) and minimal change disease (MCD) in 45.9%, 32.8% and 13.1% respectively. Hypertension was present in 58.1% and hematuria in 64.5% of cases (p value 0.001).
Conclusion: SRNS (49.18%) was the most common indication of renal biopsy and FSGS (45.9%) was the most common histopathological finding in our study. This study provides data on biopsy proven childhood renal disorders from this region.
Keywords: children; eastern India; renal biopsy; renal disorder
Full Text
Introduction
Renal diseases contribute significant morbidity and are major concern in children. Idiopathic nephrotic syndrome (NS) is a common renal disorder in children, with variation in patient characteristics in different parts of the world [1]. The incidence of idiopathic nephrotic syndrome is 1.15-16.9 per 100 000 children depending on ethnicity and region [2, 3].
Majority of these children can be diagnosed and treated without renal biopsy. However, in some situations biopsies are needed as a diagnostic resource, to determine the prognosis, and to guide the treatments as well [4]. It is difficult to determine severity of certain diseases like lupus nephritis with the clinical features alone, requiring the need of renal biopsy in all patients with systemic lupus erythematosus (SLE) nephropathy [5]. In hereditary nephropathies and in patients with persistent hematuria renal biopsy helps as an important tool determining the diagnosis and outcome [6].
It also helps in staging of IgA vasculitis with nephritis previously called Henoch -Schonlein purpura (HSP) and in diagnosis of rapidly progressing glomerulonephritis (RPGN) including its etiology sometimes [7]. Complications of percutaneous ultrasonography (USG) guided renal biopsy are negligible with the use of automated gun at present time.
The present study was done in a tertiary care teaching hospital in the state of West Bengal, India having advanced nephrology department and renal histopathology unit under the department of pathology well equipped with the facility of immunofluorescence study. Both the departments work in collaboration with pediatric medicine department to deal with the pediatric patients with complicated renal disorders. Children with difficult renal diseases are regularly referred to this Institute from different parts of the state to avail the services at free of cost.
Aim of this study was to identify the indications for renal biopsy in pediatric renal diseases and to find the clinical and histopathological correlations from our institute, a tertiary care teaching institute from eastern region of our country.
Materials and methods
This was a hospital based observational cross-section study in a tertiary care teaching institute in Kolkata, Eastern India. The period of study was one and half years from February 2021 to July 2022. The study was approved by the institutional ethics committee.
Children, aged 1-12 years, admitted in pediatric medicine with difficult to treat renal disorders and their evaluations confirmed the requirement of renal biopsy were considered as study population. Those patients satisfying the inclusion/ exclusion criteria were enrolled in this study. Sixty one (61) children were selected through typical case purposive sampling method.
The inclusion criteria were (i) Steroid resistant nephrotic syndrome (SRNS), (ii) Lupus nephritis, (iii) Acute kidney injury (AKI) patients without etiology and not improving beyond 2 weeks, (iv) HSP with Nephritic-nephrotic/ nephrotic/AKI presentation, (v) Post infectious glomerulonephritis with low C3 persisted beyond 12 weeks (vi) RPGN.
The exclusion criteria were parents not giving consent, patients with bleeding diathesis and deranged clotting parameters, uncontrolled hypertension, acute pyelonephritis, solitary kidney, severe anemia.
Method of data collection and interpretation
Thorough history was taken including information from previous medical records. Clinical examinations and relevant laboratory investigations done. Percutaneous USG guided renal biopsy was done with semi-automated gun under sedation and/or local anaesthesia in the Department of Nephrology and the biopsied tissue processing and examination was done in the Department of Pathology with light microscopy and immunofluorescence microscopy. The data were recorded in the prestructured study proforma for statistical analysis.
Definitions and parameters
Nephrotic syndrome (NS): NS is defined as the massive proteinuria (>40mg/m2/24 hour) hypoalbuminemia (serum albumin <3 g/dl), generalized edema [8].
SRNS: Absence of remission in a case of nephrotic syndrome despite adequate steroid therapy for 4 weeks [9].
RPGN: It is clinically characterized by rapidly progressive glomerulonephritis and profound loss of renal function. RPGN is the clinical course of several diseases which have crescents >50% as an underlying finding in histopathology [10].
Hematuria: Hematuria can be either microscopic or gross. Microscopic hematuria is defined as >5 RBC per high power field in a centrifuged sample of 10 ml freshly voided urine [11].
AKI: Increase in serum creatinine by ≥ 0.3 mg/dL from baseline within 48 hr; or Increase in serum creatinine to ≥ 1.5 times baseline within the prior 7 days; or urine volume ≤ 0.5 mL/kg/hr for 6 hours [12].
Statistical analysis
For statistical analysis data were compiled into Microsoft excel spreadsheet and then analyzed by SPSS version 22. Data had been summarized as mean and standard deviation for numerical variables and count and percentages for categorical variables. Comparison of proportions/percentages in categorical variables between 2 groups was done by Fisher’s exact test and in case of 2 or more groups was done by Kruskal Wallis test. P value ≤0.05 was considered to be statistically significant.
Result
Demographic data
Mean age of cases was 6.77± 3.42 years. Age range was 1-12 years. Out of the 61 cases, males were 31 and females were 30 in numbers.
Clinical presentation/ Indications
The most common clinical diagnosis was SRNS (n=30, 49.18%), followed by SLE (n=18, 29.51%). Others were AGN (n=10, 16.39%), and AKI, HSP and RPGN (each comprised n=1, 1.64 %) (Figure 1).
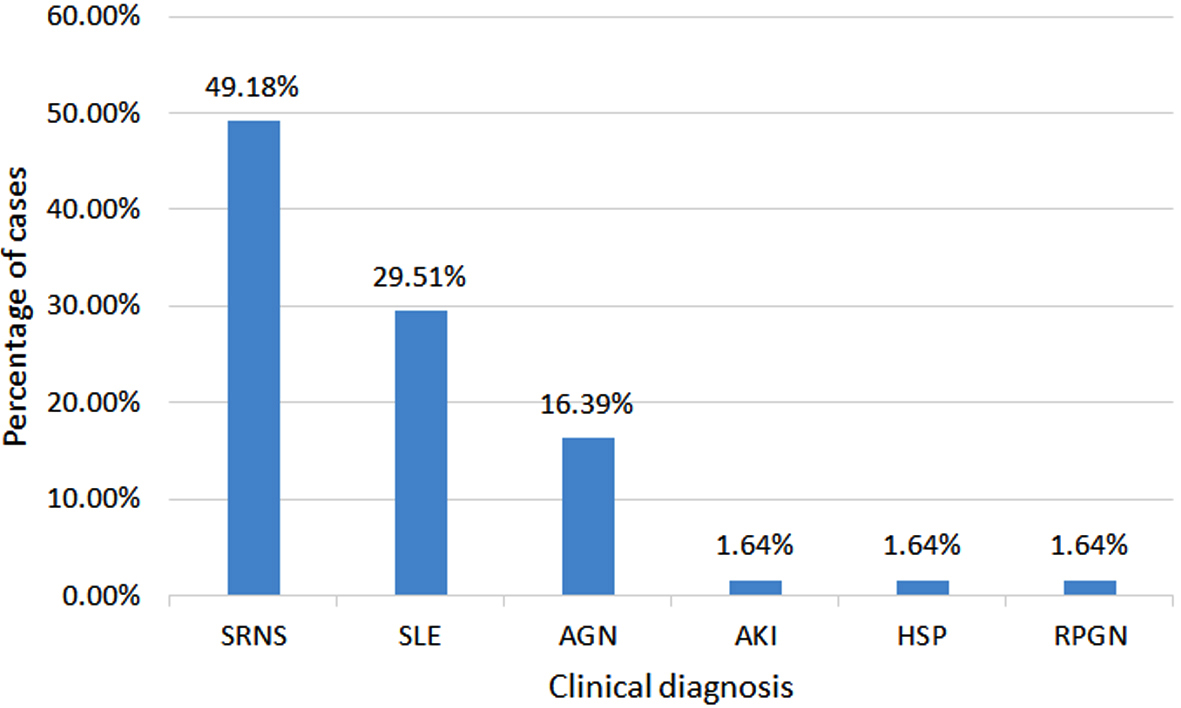
Figure 1: Distribution of cases according to indication for renal biopsy (n=61).
Histopathological diagnosis
Out of the 61patients, mean number of Glomeruli examined was 24.5 ± 11.68 (Range: 11-56). The most common histopathology (HP) was FSGS (n=28, 45.9%), followed by MPGN (n=20, 32.8%), MCD (n=8, 13.1%), crescentic glomerulonephritis (n=3, 4.9%), and IgA nephropathy and membranous glomerulonephritis (comprised n=1, 1.6% each) (Table 1).
Table 1: Distribution of cases according to renal histopathology (HP) (n=61).
|
Renal histopathology
|
Frequency
|
Percent
|
|
Focal segmental glomerulosclerosis
|
28
|
45.9
|
|
Membrano proliferative glomerulonephritis
|
20
|
32.79
|
|
Minimal change disease
|
8
|
13.1
|
|
Crescentic glomerulonephritis
|
3
|
4.91
|
|
Membranous glomerulonephritis
|
1
|
1.6
|
|
IgA nephropathy
|
1
|
1.6
|
|
Total
|
61
|
100.0
|
Clinical presentation of cases and correlation with histopathology (Table 2).
SRNS: Among the SRNS (n=30) patients, 73.33% (n=22) patients had FSGS, 23.33% (n=7)) patients had MCD, and 3.33% (n=1) had MPGN lesions.
SLE: Among the SLE (n=18) patients, 83.33% (n=15) had MPGN (p value, 0.001), 11.1% (n=2) had FSGS and 5.5% (n=1) had lesion as membranous. Lupus staging of one case could not be determined. Out of the rest 17 SLE cases, stage III and IV were in 35.3% each, 23.5% were of stage II, and only 5.9% of stage V.
AGN: Among patients with AGN (n=10), 30% (n=3) had lesions as FSGS, 30% (n=3) as MPGN, 20% (n=2) patients had lesions as Crescentic GN and 10% (n=1) each had MCD and IgA lesions.
Table 2: Distribution of cases according to clinical diagnosis and renal histopathology (n=61).
|
Clinical hiagnosis
|
Renal histopathology
|
Total
|
|
FSGS
n(%)
|
MPGN
n(%)
|
MCD
n(%)
|
Membranous
n(%)
|
Crescentric
n(%)
|
IgA
n(%)
|
|
SRNS
|
22(78.57%)
|
1(5%)
|
7(87.5%)
|
0(0%)
|
0(0%)
|
0(0%)
|
30(49.18%)
|
|
SLE
|
2 (7.14%)
|
15(75%)
|
0(0%)
|
1(100%)
|
0(0%)
|
0(0%)
|
18(29.51%)
|
|
AGN
|
3(10.71%)
|
3(15%)
|
1(12.5%)
|
0(0%)
|
2(66.67%)
|
1(100%)
|
10(16.39%)
|
|
HSP
|
0(0%)
|
1(5%)
|
0(0%)
|
0(0%)
|
0(0%)
|
0(0%)
|
1(1.64%)
|
|
RPGN
|
0
|
0(0%)
|
0(0%)
|
0(0%)
|
1(33.33%)
|
0(0%)
|
1(1.64%)
|
|
AKI
|
1(3.57%)
|
0(0%)
|
0(0%)
|
0(0%)
|
0(0%)
|
0(0%)
|
1(1.64%)
|
|
Total
|
28(100%)
|
20(100%)
|
8(100%)
|
1(100%)
|
3(100%)
|
1(100%)
|
61(100%)
|
Hypertension was present among 59% (n=36) children. Hematuria was present in 65.6% (n=40) children (p value 0.001). Presence of hematuria in FSGS group in comparison with non- FSGS group was also significant (p value 0.02). The combined presence of both hypertension and hematuria in patients with FSGS lesions were also found significant (p value 0.006) in comparison with non- FSGS group.
Discussion
The introduction of renal biopsy is one of the major events in upgrading the knowledge in nephrology. Nowadays, percutaneous renal biopsy is the most common safe method for obtaining tissue from the kidney under USG guidance.
In our study, mean age of patients was 6.77± 3.42 years (range 1 – 12 years). Similar type of studies were done by Priyadarshini et al [13], where the mean age was 5.94 years (range 4 months – 14 years), and Yadav et al [14], where the mean age was 7.9 years.. Mean age was higher in other studies. In the study by Mahapatra et al [15] mean age was 12.8 years (children age ≤ 18year), and was 9.54 years (range 1-18 years) in the study done by Printza et al [16].
Males and females were almost equal in numbers in our study, which was similar to the study done by Amatya et al [17]. However, male predominance was documented as 62.97% in studies by Priyadarshini et al [13] and male to female ratio was 1.5 in study done by Mahapatra et al [15].
The most common indication of renal biopsy was SRNS (49.18%), followed by SLE (29.51%) and AGN (16.39%), in our study. In the study conducted by Saca et al [18] the most common indication for biopsy in children was also SRNS. In a study conducted by Imtiaz S et al [19], nephrotic syndrome was the most common indication followed by acute nephritic syndrome, and, in their study done by Mahapatra et al [15], NS was also the leading indication for renal biopsy.
In our study, FSGS (45.9%) was the most common histopathological findings, followed by MPGN (32.78%) and MCD (13.1%).
Among SRNS patients (n=30), in this study, 73.33 % (n=22) patients had FSGS and 23.33% (n=7) had MCD lesions. Similar to our observation, Asinobi et al [20] documented FSGS (in 59%) as most common finding in SRNS patients followed by MCD (in 17.6%). Gulati et al [21] in their study among SRNS patients observed FSGS (in 59%) followed by MCD (in 17.6%) and in the study done by Saca et al [18], the most common histopathological findings in renal biopsy was FSGS (19%) followed by MCD (13.8%). Kari et al [22] also observed FSGS as the major lesion (39%) among SRNS patients.
Among the SLE (n=18, 29.51%) patients, the mean age was 9.4 ± 2.25 years (ranged 5 to 12 years). Lowest age of SLE patient was 5 years.
SLE patients had histopathological lesions as MPGN in 83.33% (n=15) and FSGS in 11.1% (n=2). The presence of MPGN lesion in SLE patient was significant (p value < 0.05). Majority of SLE patients had Class III and Class IV lesions, each comprising 35.5% (n=6), followed by Class II (23.5%, n=4) and the remaining 5.9% (n=1) case belonged to Class V.
Seema et al [23] conducted a study on pediatric Lupus nephritis where no child was less than 5 years as in our study and the most common HP lesion in SLE was of Class IV (in 63% cases).
In another study, Al Mayouf et al [24] documented proliferative glomerulonephritis (class III and IV) in 64.3% followed by membranous nephritis in 27.4% which was almost similar to our observations.
Hematuria was present in 64.5% (n=40) cases, (p value 0.001) in our cohort. Presence of hematuria in FSGS group was significant in comparison with non- FSGS group (p value 0.02). In a study on SRNS patients by Azhar et al [25], 31.1% children had microscopic hematuria. In their study, Gulati et al [21] also documented non-MCD children had a significantly higher prevalence of micro-hematuria than MCD children, similar to the finding in our study.
It was a single center study with small sample size and all children belonged to same ethnicity. Our conclusion may not be held true universally. Secondly, electron microscopy was not done in our study due to non-availability of the facility in our study place so diagnosis of MCD in HP was indirect showing no changes in light and IF microscopy.
Conclusion
Presence of haematuria was found to be an important predictor for FSGS among SRNS patients in this study. Majority of pediatric SLE associated nephropathy in this study were advanced class (III and IV) of proliferative GN. Our study re-emphasized SRNS as most common indications for renal biopsy in children. This study would provide data on biopsy proven childhood renal disorders from this region of our country.
Acknowledgement
All the faculty members of Nephrology and Pathology Department of our institute.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Kaddah A, Sabry S, Emil E, El-Refaey M. Epidemiology of primary nephrotic syndrome in Egyptian children. J Nephrol. 2012; 25:732–737.
[2] Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018; 392:61-74. Erratum in: Lancet. 2018; 392:282.
[3] Banh THM, Hussain-Shamsy N, Patel V, Vasilevska-Ristovska J, Borges K, et al. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J American Soc Nephrol. 2016; 11:1760–1768.
[4] Rico MP, Rodríguez CCI, Fernandez HM, González CLS, Prado AOL, at al. Characterization and Etiopathogenic approach of pediatric renal biopsy patients in a Colombian Medical Center from 2007-2017. Int J Nephrol. 2018; 2018:9603453.
[5] Prasad D. Glomerulonephritis associated with systemic lupus erythematosus. In: Kliegman RM, St Geme III JW, Blum NJ, Tasker RC, Shah SS, Wilson KM, Behrman RE, editors. Nelson Textbook of Pediatrics.21st ed. Philadelphia: Elsevier. 2019; pp.2729–2731.
[6] Bagga A, Srivastava RN. Diagnostic evaluation. In: Bagga A, Srivastava RN, editors. Pediatric nephrology. 6th ed. New Delhi: Jaypee. 2016; pp.18–41.
[7] Prasad D. Henoch-Schonlein purpura nephritis. In: Kliegman RM, St Geme III JW, Blum NJ, Tasker RC, Shah SS, Wilson KM, Behrman RE, editors. Nelson Textbook of Pediatrics. 21st ed. Philadelphia: Elsevier. 2019; pp.2731–2732.
[8] Sinha A, Bagga A, Banerjee S, Mishra K, Mehta A, et al. Steroid sensitive nephrotic syndrome: Revised guidelines. Indian Pediatr. 2021; 58:461–481.
[9] Bagga A. Management of Steroid Resistant Nephrotic Syndrome. Indian Soc Pediat Nephrol. 2009; 46:35–45.
[10] Flores FX. Rapidly Progressive (Crescentic) glomerulonephritis. In: Kliegman RM, St Geme III JW, Blum NJ, Tasker RC, Shah SS, Wilson KM, Behrman RE, editors. Nelson Textbook of Pediatrics.21st ed. Philadelphia: Elsevier. 2019; pp.2727–2728.
[11] Fransisco XF. Clinical evaluation of the child with hematuria. In: Kliegman RM, St Geme III JW, Blum NJ, Tasker RC, Shah SS, Wilson KM, Behrman RE, editors. Nelson Textbook of Pediatrics. 21st ed. Philadelphia: Elsevier. 2019; pp.2718–2720.
[12] Devaranjan P. Acute kidney injury. In: Kliegman RM, St Geme III JW, Blum NJ, Tasker RC, Shah SS, Wilson KM, Behrman RE, editors. Nelson Textbook of Pediatrics.21st ed. Philadelphia: Elsevier. 2019; pp.2769–2774.
[13] Priyadarshuni L, Pradhan SK. Pattern of renal histopathological findings in children: A single center study. J Clin Diag Res. 2019; 13:SC01–SC04.
[14] Yadav S, Kandalkar B. Epidemiology of pediatric renal diseases and its histopathological spectrum - A single-center experience from India. Saudi J Kidney Dis Transpl. 2021; 32:1744–1753.
[15] Mohapatra A, Kakde S, Annapandian VM, Valson AT, Duhli N, et al. Spectrum of biopsy proven renal disease in South Asian children: Two decades at a tropical tertiary care centre. Nephrology. 2018; 23:1013–1022.
[16] Printza N, Bosdou J, Pantzaki A, Badouraki M, Kollios K, et al. Percutaneous ultrasound-guided renal biopsy in children: a single centre experience. Hippokratia. 2011; 15:258–261.
[17] Amatya M, Pant AD. Clinical and histopathological study of renal biopsy in Nepalese children: A single center experience. PLoS One. 2022; 17:e0276172.
[18] Saca E, Hazza I, El-Imam O, Kawar M. Spectrum of biopsy-proven renal disease in the pediatric age group at King Hussein Medical Center. JRMS. 2007; 14:34–37.
[19] Imtiaz S, Nasir K, Drohlia MF, Salman B, Ahmad A. Frequency of kidney diseases and clinical indications of pediatric renal biopsy: A single center experience. Indian J Nephrol. 2016; 26:199–205.
[20] Asinobi AO, Ademola AD, Okolo CA, Yaria JO. Trends in the histopathology of childhood nephrotic syndrome in Ibadan Nigeria: preponderance of idiopathic focal segmental glomerulosclerosis. BMC Nephrol. 2015; 16:213.
[21] Gulati S, Sengupta D, Sharma RK, Sharma A, Gupta RK, et al. Steroid resistant nephrotic syndrome: role of histopathology. Indian Pediatr. 2006; 43:55–60.
[22] Kari JA, Halawani M, Mokhtar G, Jalalah SM, Anshasi W. Pattern of steroid resistant nephrotic syndrome in children living in the kingdom of Saudi Arabia: a single center study. Saudi J Kidney Dis Transpl. 2009; 20:854–857.
[23] Seema HS. A clinicopathological study of pediatric lupus nephritis in tertiary hospital of Bangalore. Int J Cur Res Rev. 2015; 7:44–47.
[24] Al-Mayouf SM, Al Ameer A, Alfattani A, Alsonbul A. Outcome of childhood lupus nephritis in Saudi children. Saudi J Kidney Dis Transpl. 2017; 28:1015–1020.
[25] Azhar A, Ikram M, Amer S. Histological pattern of steroid resistant nephrotic syndrome in children: A single centre study. J Med Sci. 2011; 19:98–101.