Full Text
Introduction
In the 1920s, Sir Berkeley Moynihan described acute pancreatitis as “the most terrible of all intra-abdominal calamities”. At the time, his approach to managing this severe condition was centered around immediate surgical intervention [1]. The goal was to remove toxic substances that were believed to accumulate in the peritoneal cavity during an acute pancreatitis attack. This method of early surgical intervention quickly became the standard treatment protocol and was widely adopted by medical centers worldwide. For nearly two decades, this aggressive surgical approach was the cornerstone of acute pancreatitis management [2].
However, by the 1940s, the medical community began to observe that patients undergoing surgical treatment for acute pancreatitis experienced higher mortality rates compared to those managed more conservatively. This realization led to a significant shift in treatment strategies, as the once-dominant surgical approach was gradually replaced by a more conservative management protocol [3]. The new protocol emphasized non-surgical interventions, including nasogastric decompression to alleviate gastric pressure, intravenous fluid therapy to maintain hydration, opiate analgesia for pain relief, and the administration of atropine to reduce gastrointestinal secretions. This conservative approach, focusing on supportive care rather than surgical intervention, became the standard of care and has persisted in various forms to this day [4].
Reginald Fitz, a pivotal figure in the early understanding of pancreatitis, laid the groundwork for modern approaches to the disease. Despite the passage of time and advancements in medical science, our understanding of acute pancreatitis has not dramatically evolved beyond Fitz's initial descriptions [5]. While there have been improvements in patient outcomes, particularly due to advancements in critical care and supportive therapies, there remains a significant gap in the development of specific medical or surgical interventions that can effectively halt the process of pancreatic autodigestion and the resulting inflammatory cascade [6].
Recent research has explored the underlying mechanisms of acute pancreatitis, with particular focus on the acinar cells of the pancreas. These cells are responsible for producing digestive enzymes, and it is believed that a disruption in the normal stimulus-secretion coupling within these cells may play a key role in the development of pancreatitis. This disruption could lead to the premature activation of digestive enzymes within the pancreas, causing the organ to begin digesting itself and triggering a severe inflammatory response [7]. Although these insights have provided a deeper understanding of the disease's pathogenesis, they have yet to translate into effective therapeutic interventions. Nonetheless, it is hoped that future research building on these findings may eventually lead to new treatment modalities capable of addressing the core mechanisms of acute pancreatitis [8].
A critical aspect of managing acute pancreatitis is the early assessment of disease severity, which is crucial for several reasons. First, accurately predicting the severity of pancreatitis early in the disease course allows clinicians to stratify patients based on their risk of developing complications [9]. This stratification is particularly important in the context of clinical trials, where identifying high-risk patients can improve the evaluation of potential treatments. Additionally, early identification of patients at risk for severe complications enables healthcare providers to implement more aggressive management strategies before those complications fully develop, potentially improving outcomes [10].
Despite the clear importance of early severity assessment, finding reliable predictors for the severity of acute pancreatitis has proven to be challenging. Various biochemical markers, imaging techniques such as contrast-enhanced computed tomography (CT), and multiple clinico-biochemical scoring systems have been employed in an attempt to gauge disease severity. However, an ideal prognostic tool—one that is simple, cost-effective, routinely available, and highly accurate-has yet to be identified [11].
The pathogenesis of organ dysfunction in acute pancreatitis is closely linked to systemic inflammation, which triggers a cascade of events including cytokine activation, hypercoagulation, and microvascular thrombosis. One potential mechanism for this organ dysfunction involves the release and activation of numerous proinflammatory cytokines, which lead to a hypercoagulable state and subsequent microvascular thrombosis [12]. Biomarkers associated with coagulation, such as D-dimer, may therefore serve as useful predictors of disease severity. D-dimer, a specific indicator of secondary fibrinolysis, has shown promise as a predictive marker for the course of acute pancreatitis, offering good sensitivity in assessing the likelihood of severe disease [13].
The aim of this study is to evaluate whether elevated D-dimer levels can serve as a reliable tool for predicting the severity and clinical outcomes in patients with acute pancreatitis. By assessing the correlation between D-dimer levels and disease progression, the study seeks to determine the utility of this biomarker in identifying high-risk patients, thereby aiding in more accurate prognosis and potentially guiding early therapeutic interventions.
Materials and methods
This prospective, observational study conducted at Jubilee Mission Medical College and Research Institute, Thrissur after getting ethical committee approval. It was included 60 patients diagnosed with acute pancreatitis based on clinical, biochemical, and imaging criteria. Inclusion criteria ware patients aged over 18 years, presenting within 72 hours of symptom onset, and providing consent. Exclusion criteria include patients under 18, those presenting after 72 hours, and those refusing participation. ROC analysis and diagnostic evaluations such as sensitivity, specificity, PPV, NPV, and accuracy was employed to assess the predictive value of elevated D-dimer levels for disease severity and outcomes. The study was done from October 2022 to March 2024, for a period of 18 months.
Results
In this study of 60 participants, 36.7% (22) were aged 21-30, 8.3% (5) were 31-40, 25% (15) were 41-50, and 15% (9) each were in the 51-60 and >60 age groups. The sample included 63.3% males (38) and 36.7% females (22). The majority of participants were younger, with a significant proportion in the 21-30 age group, and males predominated in the study population as shown in table 1.
Table 1: D-dimer values and Atlanta classification (Cross tabulation).
|
D-dimer values (ng/L)
|
Atlanta classification
|
Total
|
|
MILD
|
MSAP
|
SAP
|
|
≤1871
|
Count
|
29
|
3
|
1
|
33
|
|
%
|
87.9%
|
9.1%
|
3.0%
|
100%
|
|
1871-2528
|
Count
|
2
|
6
|
0
|
8
|
|
%
|
25.0%
|
75.0%
|
0.0%
|
100%
|
|
≥2528
|
Count
|
4
|
9
|
6
|
19
|
|
%
|
21.1%
|
47.4%
|
31.6%
|
100%
|
|
Total
|
Count
|
35
|
18
|
7
|
60
|
|
%
|
58.3%
|
30.0%
|
11.7%
|
100%
|
D-dimer values of ≤1871 ng/L were primarily associated with mild acute pancreatitis. Among the 7 cases of severe acute pancreatitis (SAP), 6 had D-dimer values of ≥2528 ng/L, indicating a strong correlation between higher D-dimer levels and the severity of the condition. This suggests that elevated D-dimer levels may serve as a predictive marker for more severe forms of acute pancreatitis as shown in table 2.
Table 2: Association between D-dimer and need for hemodialysis.
|
D-dimer
|
Need for hemodialysis
|
χ2 value
|
p value
|
|
Yes
|
No
|
|
n
|
%
|
n
|
%
|
|
≥1871 (n=27)
|
4
|
14.8
|
23
|
85.2
|
3.126
|
0.077
|
|
< 1871 (n=33)
|
0
|
0
|
33
|
100
|
Among patients with D-dimer values >1871 ng/L, 4 required haemodialysis, while 23 did not. Although there was a difference in the need for haemodialysis based on D-dimer levels, the association was not statistically significant, with a p-value of 0.077. This suggests that while higher D-dimer levels may be associated with a greater need for haemodialysis, the evidence is not conclusive.
The cut-off value for D-dimer to predict complications in acute pancreatitis was 1871 ng/L, with an AUC of 0.870 (95% CI, 0.778-0.962). It showed 87.5% sensitivity, 83.3% specificity, a positive predictive value of 77.78%, a negative predictive value of 90.90%, and 85% accuracy. Patients with D-dimer levels below 1871 ng/L mostly developed mild acute pancreatitis, indicating D-dimer as a reliable predictor of severity and complications as shown in figure 1.
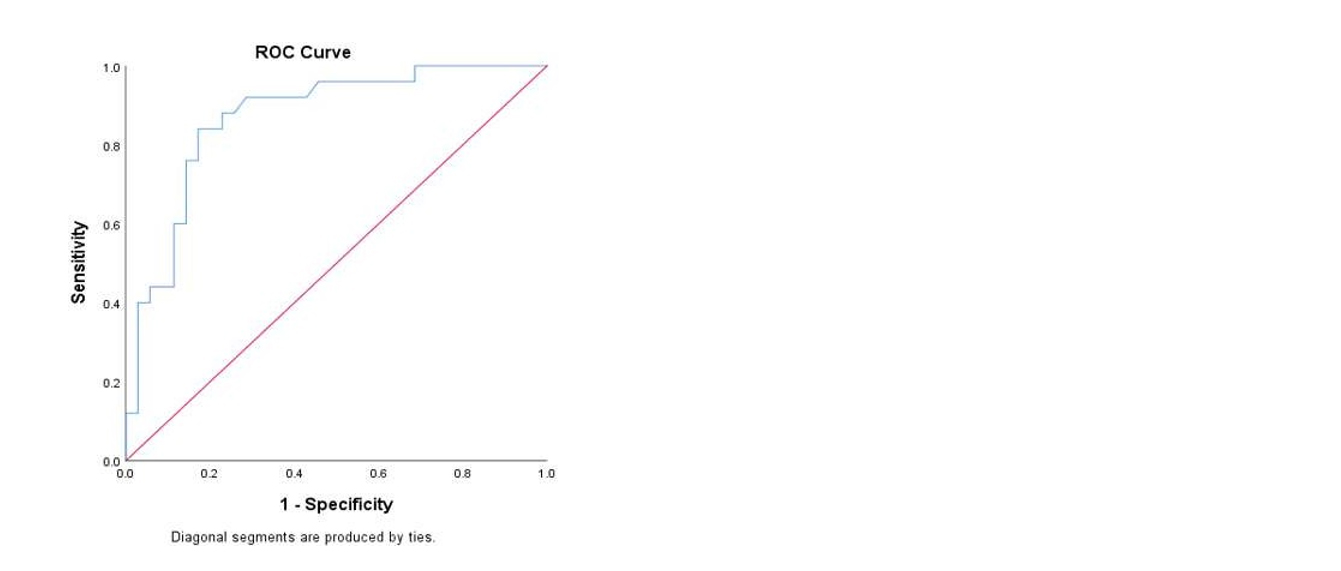
Figure 1: The ROC curve.
Among patients with D-dimer values >1871 ng/L, 66.7% (18 patients) required oxygen support, compared to only 9.1% (3 patients) with D-dimer values <1871 ng/L. Conversely, 33% (9 patients) with D-dimer >1871 ng/L did not need oxygen support, while 30 patients (91%) with values <1871 ng/L did not require it. This difference is statistically significant, indicating that higher D-dimer levels are strongly associated with the need for oxygen support in acute pancreatitis as shown in table 3.
Table 3: Association between D-dimer and need for oxygen support.
|
D-dimer
|
Need for oxygen support
|
χ2 value
|
p value
|
|
Yes
|
No
|
|
n
|
%
|
n
|
%
|
|
≥1871 (n=27)
|
18
|
66.7
|
9
|
33.3
|
21.638
|
0.00
|
|
< 1871 (n=33)
|
3
|
9.1
|
30
|
90.9
|
Among the 21 patients requiring oxygen support, 33% (7 patients) needed High Flow Nasal Oxygen, 29% (6 patients) required a face mask, 14% (3 patients) used nasal prongs, and 24% (5 patients) required mechanical ventilation. This distribution highlights the varying levels of respiratory support needed among patients, with a significant proportion requiring more intensive interventions like High Flow Nasal Oxygen and mechanical ventilation as shown in figure 2.
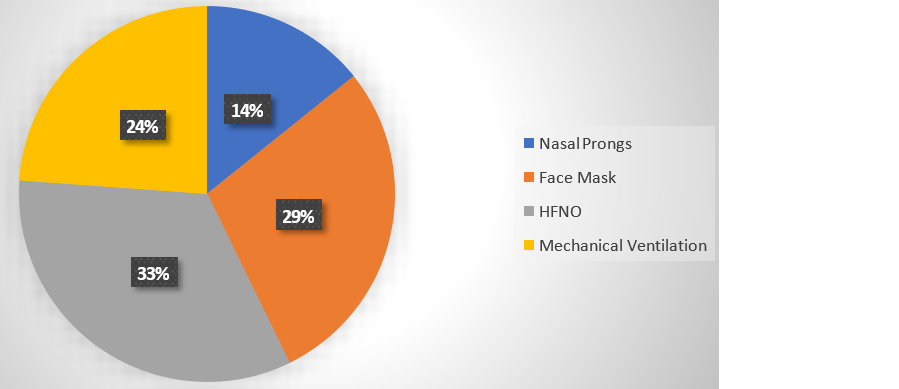
Figure 2: Depicts type of oxygen support needed for patients with respiratory complication.
Among the 24 patients who developed complications, 16.7% (4 patients) experienced renal complications, and 58.3% (14 patients) had respiratory complications. None of the patients developed isolated cardiac complications. The remaining 25% (6 patients) suffered from multisystemic complications, indicating that respiratory issues were the most common, followed by renal and multisystemic complications among those with acute pancreatitis-related complications as shown in table 4.
Table 4: Shows the type of complications of acute pancreatitis in the study group.
|
Type of complication
|
Frequency
|
Percentage
|
|
Renal
|
4
|
16.7
|
|
Respiratory
|
14
|
58.3
|
|
Cardiac
|
0
|
0
|
|
Multisystem
|
6
|
25.0
|
|
Total
|
24
|
100
|
Among the 60 cases, 66.66% (40 patients) presented with abdominal pain, making it the most common symptom. Nausea and vomiting were the chief complaints in 25% (15 cases), while 8.34% (5 cases) presented with breathing difficulty. This distribution highlights abdominal pain as the predominant symptom in the study population, followed by nausea, vomiting, and breathing difficulty as shown in figure 3.
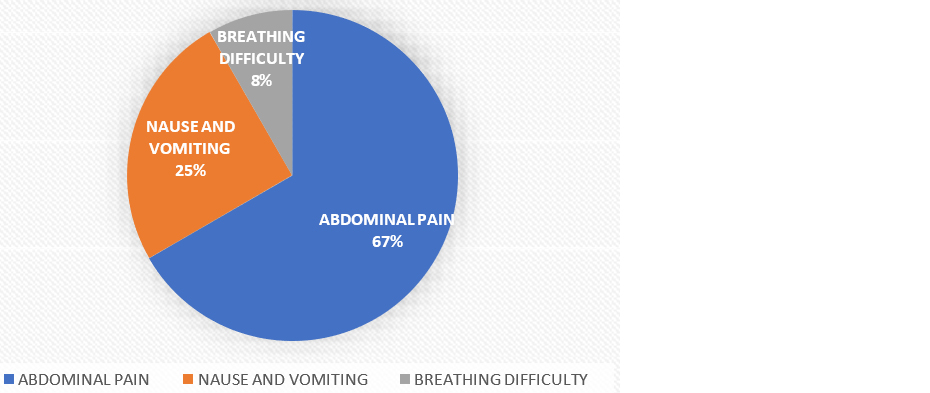
Figure 3: Shows the presenting complaints of the patients presented with acute pancreatitis.
Among the total cases, 58.3% (35 cases) were classified as mild acute pancreatitis, 30% (18 cases) as moderately severe acute pancreatitis, and the remaining 11.7% (7 cases) as severe. The classification of pancreatitis severity was based on the Atlanta Classification, highlighting the distribution of cases across different severity levels within the study population as shown in table 5.
Table 5: Shows the type of pancreatitis according to Atlanta Classification.
|
Atlanta classification
|
Frequency
|
Percentage
|
|
Mild acute pancreatitis
|
35
|
58.3
|
|
Moderately severe acute pancreatitis
|
18
|
30.0
|
|
Severe acute pancreatitis
|
7
|
11.7
|
|
Total
|
60
|
100
|
Table 6: Shows the association between d- dimer and need for ionotropic support.
|
D-dimer
|
Need for ionotropic support
|
χ2 Value
|
p value
|
|
Yes
|
No
|
|
n
|
%
|
n
|
%
|
|
≥1871 (n=27)
|
5
|
18.5
|
22
|
81.5
|
4.463
|
0.010
|
|
< 1871 (n=33)
|
0
|
0
|
33
|
100
|
In 5 of the patients with D-dimer values >1871 needed ionotropic support whereas the rest 22 did not require the same. With a p value of 0.010 it is found to be statistically significant.
Discussion
Our study found 15% of participants were aged 51-60 and over 60, consistent with Makris et al. on those older than 65 and Yu et al. on trends in the 65-74 age group. Gender distribution was 63.3% male and 36.7% female, aligning with Özdemir et al. and Brahim et al., both reporting a higher male proportion. These findings highlight the importance of age and gender as key demographic factors in clinical research [14-17].
Our study found that 58.3% of patients had alcohol-related etiology and 40% had gallstones, which correlates with Samanta et al. who observed alcoholic pancreatitis in 48.5% and gallstone disease in 32.4% of 759 patients. They also noted that gallstone pancreatitis was more prevalent in older patients with a higher female predilection. These findings are consistent with those of Mederos et al., highlighting similar trends in the etiological distribution of pancreatitis [18, 19].
Our study found that 58.3% of cases were classified as mild acute pancreatitis, 30% as moderately severe, and 11.7% as severe according to the Atlanta Classification, consistent with the findings of Özdemir et al. and Samanta et al. Additionally, we observed that 66.66% of cases presented with abdominal pain, 25% with nausea and vomiting, and 8.34% with breathing difficulty. These symptom distributions align with the findings of Makris K et al. and Özdemir et al. The consistency across these studies highlights the reliability of our observations and underscores the importance of standardized classifications and symptom assessments in pancreatitis research [14, 16, 18].
Our study found that 40% of cases developed complications, while 60% did not, aligning with the findings of Vipul et al. and Samanta et al. Vipul et al. reported a similar complication rate of 42%, while Samanta et al. observed complications in 38% of cases. These consistent findings highlight the importance of vigilant monitoring and management in patient populations at risk of developing complications [18, 20].
Our study found that 16.7% of patients developed renal complications, 58.3% had respiratory issues, and 25% had multisystemic complications, with no isolated cardiac cases. These findings align with Vipul et al. and Samanta et al., who reported similar patterns of complications, emphasizing the prevalence of respiratory and multisystemic complications in their studies [18, 20].
Our study found that a D-dimer cutoff of 1871 ng/L, with an AUC of 0.870, effectively predicted complications in acute pancreatitis, particularly indicating MSAP at 1871-2528 ng/L and SAP above 2528 ng/L. These findings align with Mederos et al. and Zhang et al., who reported similar correlations between D-dimer levels and pancreatitis severity, reinforcing its reliability as a predictive marker for complications in such cases [19, 21].
Our study found that D-dimer levels >933.5 ng/L effectively predicted complicated acute pancreatitis (CAP) and correlated with severity, ICU requirements, and APACHE II scores, similar to findings by Newton et al. and Kumar et al. highlighting its value in early diagnosis and referral decisions in resource-limited settings [22, 23].
Our study found that five patients with D-dimer values >1871 ng/L required ionotropic support, while 22 did not, with a statistically significant p-value of 0.010. This result aligns with the findings of Newton et al. and Wan J et al. both of whom also reported a significant correlation between elevated D-dimer levels and the need for ionotropic support in acute pancreatitis patients, highlighting its predictive value for severe outcomes [22, 25].
Limitations: Limitations, such as variability influenced by age, comorbidities, and acute conditions. Additionally, challenges in standardizing D-dimer measurements across laboratories complicate its clinical application. Addressing these challenges requires larger, multi-center trials to better understand D-dimer's prognostic value across diverse patient populations.
Conclusion
This study demonstrates that D-dimer can serve as a valuable marker for predicting the severity and complications of acute pancreatitis. D-dimer levels ≥1871 ng/L effectively predicted complications, with levels between 1871-2528 ng/L indicating MSAP and levels >2528 ng/L indicating SAP. Current research on D-dimer frequently depends on single-center studies with small sample sizes, potentially overlooking the comprehensive variability of D-dimer levels and their clinical significance. To achieve a thorough understanding, larger multi-center trials are crucial. Such studies would validate findings across diverse demographics, geographic locations, and clinical environments, thereby furnishing stronger evidence of the diagnostic and prognostic value of D-dimer in both acute and chronic conditions. This evidence could significantly enhance clinical decision-making.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Pradhan S, Shah J. Presence of choledocholithiasis in patients undergoing cholecystectomy for mild biliary pancreatitis. Bangladesh J Med Sci. 2016; 15:517–521.
[2] Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014; 20:13879.
[3] Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chinese J Cancer Res. 2015; 27:332.
[4] Isaias GC, Duenas C, Perales K, Juan, Andres SG, et al. Approach to patient management in critical condition. Health Sci J. 2022:1–26.
[5] Ali S, Fatima A, Arshad T. A review on the characterization, causes, and treatment of the pancreatitis disease. Int J Sci Res Sci Technol. 2017; 3:451–461.
[6] Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol. 2016; 35:153–166.
[7] Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. The Lancet Respiratory Medicine. 2021; 9:622–642.
[8] Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010; 11:85.
[9] Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scandinavian J Gastroenterol. 2010; 45:959–970.
[10] Kylänpää L, Rakonczay Jr Z, O′ Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflammat. 2012; 2012:360685.
[11] Soomro AY, Guerchicoff A, Nichols DJ, Suleman J, Dangas GD. The current role and future prospects of D-dimer biomarker. Eur Heart J Cardiovascul Pharmacother. 2016; 2:175–184.
[12] Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. The Clinical Biochemist Reviews. 2016; 37:85.
[13] Yu J, Liu C, Zhang J, Wang X, Song K, et al. Global, regional, and national burden of pancreatitis in older adults, 1990–2019: A systematic analysis for the global burden of disease study 2019. Preventive Medicine Reports. 2024; 41:102722.
[14] Özdemir BC, Gerard CL, da Silva CE. Sex and gender differences in anticancer treatment toxicity: a call for revisiting drug dosing in oncology. Endocrinology. 2022; 163:bqac058.
[15] Brahim NB. Integrated system for an automated insulin therapy for type 1 diabetes: real time evaluation of the effect of physical exercise and adjustment of the insulin dosage. Halscience. 2016. Available from: https://theses.hal.science/tel-01496776.
[16] Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A et al. Coronavirus disease 2019 and the pancreas. Pancreatol. 2020; 20:1567–1575.
[17] Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021; 325:382–390.
[18] Srivastava VK, Khanna R, Meena R, Khanna S, Sing C, et al. Serum d-dimer as a tool for assessment of severity in patients of acute pancreatitis. Int J Sci Res. 2021; 10. Available from: https://www.worldwidejournals.com/international-journal-of-scientific-research-(IJSR)/article/serum-dandndash-dimer-as-a-tool-for-assessment-of-severity-in-patients-of-acute-pancreatitis/MzQxNzk=/
[19] Zhang GQ, Wang G, Li L, Hu JS, Ji L, et al. Plasma D-dimer level is an early predictor of severity of acute pancreatitis based on 2012 Atlanta Classification. Med Sci Monit. 2019 Nov 27; 25:9019–9027.
[20] Newton MV. D-dimer as a marker of severity and prognosis in acute pancreatitis. Int J Applied Basic Med Res. 2024; 14:101–107.
[21] Kumar A, Kothagattu R. D-dimer levels in predicting the severity of acute pancreatitis. International Surgery J. 2017; 4:3993.
[22] Radha RB. Evaluation of d-dimer level in predicting the severity of acute pancreatitis and early assessment of organ failure. Bldeduacin. 2020; Available from: https://digitallibrary.bldedu.ac.in/handle/123456789/4215
[23] Wan J, Yang X, He W, Zhu H, Zhu, et al. Serum D-dimer levels at admission for prediction of outcomes in acute pancreatitis. BMC Gastroenterol. 2019; 19:67.