Full Text
Introduction
Oral cancer ranks sixth among all cancers globally and India has the most significant number of cases, one-third of the total burden of oral cancer cases globally [1]. India faces a severe oral cancer burden, with 77,000 new cases and 54,000 annual deaths [2, 3] and it primarily affects young adults, presenting in advanced stages with a low cure rate (40-50%) [1]. Squamous cell carcinoma (SCC) constitutes over 90% of oral and pharyngeal cancers [4]. Oral tongue carcinoma risks in India include tobacco use, alcohol consumption, poor oral hygiene, viral infections, and nutrient-deficient diets [1]. The most common areas involved in the tongue were the lateral borders especially at the junction of anterior 2/3rd and posterior 1/3rd [5].
The 8th edition of AJCC introduced Depth of Invasion (DOI) in staging oral cancers, crucial for prognostication and deciding elective neck dissection. DOI, distinct from tumor thickness (TT), reflects the tumor's deepest invasion and is a strong predictor of occult lymph node metastasis and disease-specific survival. MRI, ultrasound, and CT aid DOI measurement, with MRI being well-studied and reliable. Guidelines recommend considering elective neck dissection when DOI exceeds 4 mm. Achieving tumor-free margins is vital in surgery, with a 1-1.5 cm intraoperative margin recommended. Inadequate resections range from 30-85%, emphasizing the challenge in assessing margins, especially the elusive deep margin. Positive deep margins are common, leading to unnecessary adjuvant treatments and increased patient burden [6-12]. In order to mitigate the close or positive margins of the deep margin, several intraoperative assessment methods have been developed. Frozen section analysis (FSA), near-infrared fluorescence, hyperspectral imaging (HSI), optical coherence tomography (OCT), narrow-band imaging (NBI), ultrasound, computed tomography, specimen radiography, magnetic resonance imaging, and image-guided surgery are in use [13]. Frozen Section Analysis (FSA) is widely employed, with 97% of head and neck surgeons incorporating it in practice [14]. Auto-fluorescence, narrow band imaging, and optical coherence tomography are not suitable for deep-margin assessment [15]. USG is a dynamic imaging technique, using sound waves in the megahertz range. It is non-invasive and quick. USG assessment is a better predictor than manual palpation and deep margin can be assessed both in vivo and ex vivo using high-resolution USG probe [16, 17].
The aim of the study was to conduct deep margin assessment using intraoperative ultrasound in superficial oral tongue carcinomas, ensuring adequate surgical resection for early-stage oral tongue cancer minimizing loco regional recurrence risks and to correlate ultrasound measurements with histologically determined deep margin values and prevent close margins.
Methodology
The prospective observational study involving 91 diagnosed tongue malignancies at the Krishna Institute of Medical Sciences, Department of Surgical Oncology, conducted from 2020 to 2023 (two and half years). Patients provided oral and written consent after being informed about the procedure and study details. The study included biopsy verified tongue cancer and excluded patients with prior oral cavity surgery, floor of the mouth cancer not available for ultrasound investigation, extension of floor of the mouth cancer to the gingiva, those not suitable for surgery, T4 tumors, individuals under 18, and those declining to participate. All cases were studied prospectively without randomization, and the same surgeon treats all patients. Pathology specimens are reviewed by a single pathologist, ensuring consistency. The study had received approval from Institutional Ethics Committee.
During the surgical procedure and under general anaesthesia the tongue lesions of the patient were evaluated with intraoral Sonography. An intraoral transducer probe characterized by an 8 to 10 MHz ultrasonic beam Siemens Acuson NX3 with water-based eco gel and probe cover was used to assess the deep margin, Tumor thickness, margins, and other findings like lingual nodes are noted. The surgical approach was transoral in all cases and oral wash a USG probe enclosed in a sterile plastic cover was introduced into the surgical field placed on the surface of the tongue lesion and evaluated for deep margin, DOI, and any lingual nodes. This information was reconfirmed by clinical examination with palpation. Resection had proceeded in standard fashion. Wide local excision with a margin of 1.5cm is done in three dimensions. Once the resection is completed the resected tongue specimen is examined ex-vivo using the USG probe for deep-margin assessment. Applying undue pressure on the specimen may distort the deep margin and tumor thickness due to external forces. After thorough scanning in all directions and deep margin, images were obtained and values were noted, and if the deep margin was close it was revised immediately. The surgical specimen later is sent for frozen section analysis. In the frozen section laboratory, tumor was cut and gross measurements were taken and surgical margin were noted. Close or suspicious margins were taken for the frozen section. The values were noted and compared to the Final histopathology report. All the demographic details and the measured study variables were noted in the proforma and data imported into an Excel sheet and analyzed.
Results
A total of 91 patients that underwent resection using Intraoperative USG were included in the study. The majority of the patients were in the age group 41 - 60 years. As carcinoma tongue is more common in males our study has a sex distribution of 63 male and 28 females. Patients presented with T1 disease were 29, 36 patients in T2, 26 patients in T3 stage, and T4 tumors with reduced mouth opening and inability to do USG examination are excluded from the study. Data was taken in a systematic manner filling the proforma and all the data exported onto an excel sheet. Margins greater than 0.5 mm were considered free and any margin less than 0.5 mm was considered close and less than 0.1cm was considered positive.
Table 1: Paired Samples Correlations.
|
|
N
|
Correlation
|
Significance
|
|
One-Sided p
|
Two-Sided p
|
|
Pair 1
|
USG deep margin (CM) & Frozen section deep margin (CM)
|
91
|
.964
|
<.001
|
<.001
|
|
Pair 2
|
USG deep margin (CM) & Deep margin final (CM)
|
91
|
.916
|
<.001
|
<.001
|
The deep margin assessment, the main objective of the study, was assessed by the USG value noted and a mean USG deep margin was derived and it was compared to the deep margin assessed by the frozen section and final histopathological examination which is the gold standard. The mean deep margin assessed by USG - is 0.901cm and the mean deep margin assessed by the frozen section is 0.762 cm. The mean deep margin assessed by final histology is 0.748 cm.
Table 2: correlation and p-value between USG deep margin and frozen and final deep margins.
|
|
USG deep margin (Cm)
|
Frozen section deep margin (Cm)
|
Deep margin final (Cm)
|
|
N
|
Valid
|
91
|
91
|
91
|
|
Missing
|
0
|
0
|
0
|
|
Mean
|
.9011
|
.7626
|
.7484
|
|
Std. Deviation
|
.52228
|
.48526
|
.50318
|
|
Minimum
|
.10
|
.10
|
.10
|
|
Maximum
|
3.00
|
3.00
|
3.00
|
|
Percentiles
|
25
|
.5000
|
.4000
|
.4000
|
|
50
|
.8000
|
.7000
|
.6000
|
|
75
|
1.3000
|
1.0000
|
1.0000
|
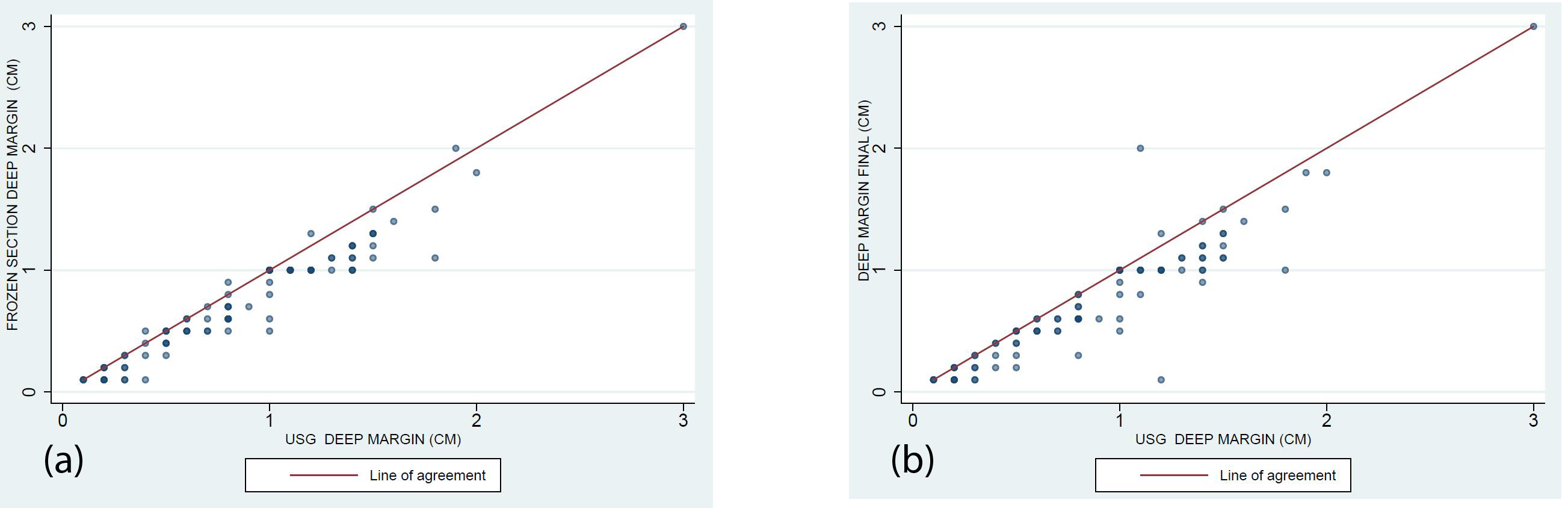
Figure 1: Scatter plot.
Scatter plot analysis shows the reliability of positive USG deep margin assessment as compared to the frozen section and final HPE assessment. (R2= 0.967).
Discussion
Surgery is the first and best choice of treatment in early-stage T1, T2, and T3 tumors of oral tongue squamous cell carcinoma [18]. Radical resection with good margins is a prerequisite for better recurrence-free, disease-free, and overall survival [19]. But the percentage of resection margins that are inadequate in oral tongue carcinoma to date is still around 30% - 80% [10]. Resection margins with tumor cells less than 1mm is considered positive margin, less than 5mm are considered close margins, and more than 5mm are considered free [20]. Obtaining negative margins is the most important factor in the surgeon's hands. The most common margin that is usually close or positive is the deep margin [21]. Most of the patients with inadequate resections with close and positive margins require adjuvant therapy in the form of radiation and chemoradiation which are associated with increased morbidity and decreased quality of life and additional increased financial burden [22].
Margin assessment after tumor resection is critical and it's the only prognostic factor in the surgeon's hands and is very challenging. Many methods are followed from gross examination, palpation, frozen section, image-guided surgery like Ultrasound, spectroscopy, fluorescence imaging, and final histopathological examination [13]. The most ideal technique for guidance is that which provides a direct and quick response of the complete mucosal and deep margins with microscopic accuracy during resection Frozen section analysis is the most commonly followed intraoperative assessment of margins after tongue resection surgery [15]. This frozen section analysis not only widely available but has drawbacks like, it has high rates of false negatives, type of frozen section i.e., specimen-driven FSA or patient-driven FSA is different with varied results. Relocating the sample site after it is reported as close or positive is very difficult and challenging and Kerwala et al showed an error of 12mm for relocation of the sample site [13]. After resection by the time the specimen reaches the pathologist tissue is subjected to shrinkage leading to close margins. So, an ideal technique to assess deep margins or mucosal margins should be easy, cheap, widely available, and fast and should be done in the operating room. Fluorescence imaging and other optical techniques like Spectroscopy, hyperspectral imaging, optical coherence tomography, and Narrow band imaging are costly and are in evolving stages and may be promising in the future [13]. In the literature, data on deep resection margin is scarce, previous studies have indicated that USG is a promising technique in assessing the deep margin which is fast and reliable when compared to conventional methods [23]. Real-time USG guidance aids surgeons in adjusting resection margins, crucial for the small and highly functional tongue. In this study, we assessed post-tongue resection deep margins to prevent close and positive margins.
A total of 91 patients were included in the study which is a higher number when compared to the previous studies in the literature, Yoon et al in 2020 (N = 36), De Koning et al in 2020 (N=31), and Nilsson et al (N = 34). In the present study we found that most of the patients were in the age group > 41 years of age similar findings were observed by Nilsson et al where the minimum and maximum age groups in USG-assisted surgery and the conventional group were 34-86 and 27-88 years respectively. Most of the patients in our study are in the T2 stage 36/91 ie., 39.5 %. As the Baek et al group, we used the mean deep margin as an outcome measure 0.901cm+/- 5.2 mm (mean +/- SD) as the outcome measure the mean deep margin assessed by USG in our study was 0.901cm+/- 5.2 mm (mean +/- SD) and the mean deep margin assessed by the frozen section is. 0.762 cm +/- 4.8 mm. The mean deep margin assessed by final histology is 0.748 cm+/- 5.0 mm. In the present study, 78.1 % of the USG-assisted resections had clear margins similar to De Koning et al who compared 40 USG-assisted resections to 96 conventional resections and found significant improvement in deep margins in USG-assisted resections 55% vs 15%.
Higher stage T3 and T4 was a strong predictor for inadequate deep margin but they were assessed correctly by USG assessment and allowed us to revise the margins to prevent close and positive margins. On statistical analysis a strong correlation was observed between USG guided resection mean deep margin and histopathological deep margin.
We identified that USG / and histologic i.e., frozen and final histopathological deep margins are almost similar and developed a detailed insight in terms of the accuracy of the technique and its utility. USG-guided surgery for early-stage carcinoma tongue is an accessible and inexpensive technique and provides a good overview of deep and submucosal margins, moreover pre-excisional USG for carcinoma tongue gives us an insight into the depth of invasion, tumor extent, and presence of lingual nodes without any adverse events. In this study, we have shown that USG has good accuracy in assessing the deep margin in carcinoma tongue patients, ensuring that it is a reliable method for surgical guidance. Preoperatively, assessing DOI faced challenges due to issues like gag reflex, pain, and movement limitations. Additionally, the occurrence of lingual nodes was rare, accounting for around 2% with only a few case reports. Lingual nodes are an inconstant lymph node group which cannot be implied for every tongue cancer patient. Anatomically-obtained incidences of LLN range from 8.6% to 30.2% [24].
Intraoperative USG guides optimal tumor resection, ensuring adequate margins while preserving essential native tongue function. While lacking long-term follow-up, we anticipate favorable outcomes, potentially minimizing adjuvant therapy-related morbidity. Future studies with control groups can assess avoidance of radiation therapy and impact on survival. Our study informs a USG criterion for deep margin assessment during tongue cancer resection.
Criteria for USG assessment of deep margin
Ultrasound-guided assessment is suitable for early-stage tumors, reducing operative time. Challenging in advanced cases, ex vivo assessment is possible post-resection. Operator-dependent, it requires a high-resolution linear probe with minimal transducer pressure. Lack of a control group and long-term follow-up are study limitations. Current results suggest favorable outcomes with clear margins in USG-guided resections.
Conclusion
In conclusion, USG guided resection of early tongue cancers is a technique that is able to increase the frequency of free margins and decrease the close margin and positive margin frequency when compared to conventional treatment. USG being noninvasive is a very fast method in comparison to the frozen section and seems to be a promising technique. Larger studies are needed with control groups to possibly confirm a statistically significant difference in adequate deep resection margin and improvement in DSS and Quality of life in future.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int. 2020; 1:100046.
[2] Laprise C, Shahul HP, Madathil SA, Purakkal AST, Castonguay G, et al. Periodontal diseases and risk of oral cancer in Southern India: Results from the HeNCe Life study. Int J Cancer. 2016; 139:1512–1519.
[3] Keshani F, Jalayer S, Esfahani M. Prevalence of oral squamous cell carcinoma cases for ten years in Qazvin province (2003-13). J Qazvin Univ Med Sci. 2017; 21:95–99.
[4] Chimenos-Küstner E, Marques-Soares MS, Schemel-Suárez M. A etiopathology and prevention of oropharyngeal cancer. Semergen. 2019; 45:497–503.
[5] Khalesi S, Abbasi A, Razavi SM. Evaluating the clinicopathologic parameters of tongue squamous cell carcinoma based on its local distribution. Adv Biomed Res. 2023; 12:71.
[6] Jemal A, Thomas A, Murray T. Cancer statistics, 2002. CA Cancer J Clin. 2002; 52:23.
[7] D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015; 373:521–529.
[8] Kirtane K, Rodriguez CP. Postoperative combined modality treatment in high risk resected locally advanced squamous cell carcinomas of the head and neck (HNSCC). Front Oncol. 2018; 8:588.
[9] Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–99.
[10] Smits RWH, Koljenović S, Hardillo JA, Hove IT, Meeuwis CA, et al. Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016; 38:e2197–e2203.
[11] Weijers M, Snow GB, Bezemer DP, Wal JEV, Waal IVD. The status of the deep surgical margins in tongue and floorof mouth squamous cell carcinoma and risk of local recurrence; an analysis of 68 patients. Int J Oral Maxillofac Surg. 2004; 33:146–149.
[12] Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005; 41:1034–1043.
[13] Koning SGB, Schaeffers AWMA, Schats W, van den Brekel MWM, Ruers TJM, Karakullukcu MB. Assessment of the deep resection margin during oral cancer surgery: A systematic review. Eur J Sur Oncol. 2021; 47:2220–2232.
[14] Kerawala C, Ong KW. Relocating the site of frozen sections – is there room for improvement? Head Neck. 2000; 23:230–232.
[15] Bulbul MG, Zenga J, Tarabichi O, Parikh AS, Sethi RK, et al. Margin practices in oral cavity cancer resections: survey of American head and neck society members. Laryngoscope. 2021; 131:782–787.
[16] Baek CH, Son YI, Jeong HS, Chung MK, Park KN, et al. Intraoral sonography-assisted resection of T1-2 tongue cancer for adequate deep resection. Otolaryngol Head Neck Surg. 2008; 139:805–810.
[17] Koning KJ, Es RJJ, Klijn RJ, Breimer GE, Dankbaar JW, et al. Application and accuracy of ultrasound-guided resections of tongue cancer. Oral Oncology. 2022; 133:106023.
[18] Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015; 33:3269–3276.
[19] Jain PV, Sharan R, Manikantan K, Clark GM, Chatterjee S, et al. Redefining adequate margins in oral squamous cell carcinoma: outcomes from close and positive margins. Eur Arch Otorhinolaryngol. 2020; 277:1155–1165.
[20] Müller S, Boy SC, Day TA, Magliocca KR, Richardson MS, et al. Data set for the reporting of oral cavity carcinomas: Explanations and recommendations of the guidelines from the international collaboration of cancer reporting. Arch Pathol Lab Med. 2019; 143:439–446.
[21] Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003; 32:30–34.
[22] Jehn P, Stier R, Tavassol F, Dittmann J, Zimmerer R, et al. Physical and psychological impairments associated with mucositis after oral cancer treatment and their impact on quality of life. Oncol Res Treat. 2019; 42:342–349.
[23] Nilsson O, Knutsson J, Landström FJ, Magnuson A, Beckerath M. Ultrasound-assisted resection of oral tongue cancer. Acta Otolaryngol. 2022; 142:743–748.
[24] Nishio N, Fujimoto Y, Hiramatsu M, Yamamoto Y, Sone M. Sonographic detection of a lingual node metastasis from early squamous cell cancer of the tongue. J Clin Ultrasound. 2018; 46:69–72.