Full Text
Introduction
Vulvovaginitis is an inflammation of vaginal epithelium characterized by discharge, itching and pain [1]. Vaginitis can be caused by microenvironment variation due to physiological changes (hormonal imbalance) or infections [2, 3] . The most common causes of infectious vaginitis are bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and trichomoniasis [1, 4]. The overall prevalence of vaginitis is 30% nationwide and it affects approximately 1-14% of women of reproductive age group [5]. In India, the prevalence of bacterial vaginosis is 15-46%, vaginal candidiasis is 25- 60% and Trichomoniasis is about 6-10% [1, 5, 6, 7].
BV is a dysbiosis which is characterised by replacement of resident flora of vagina (Lactobacilli) with anaerobic microorganisms such as Gardnerella vaginalis and Mobiluncus spp., leading to increase in the vaginal pH [1, 8]. It usually presents as increased vaginal discharge that is greyish-white in colour with fishy odor. Various factors can contribute to development of bacterial vaginosis, like multiple sexual partners, sexual activity at young age, douching, smoking, use of over-the-counter intravaginal hygiene products and high stress levels [2, 7, 8]. In young females, bacterial vaginosis is a common cause of vaginitis which even after treatment can often cause recurrence in 80% patients [1, 2, 8]. VVC is another common cause of vaginal discharge in which Candida albicans is elicited as the most common etiological agent [1, 2, 9]. In India, the prevalence of VVC is higher wherein female population suffers from genital candidiasis at least once in their lifetime and 50% of females suffer from recurrent candidiasis [1, 2, 7, 10]. Although Candida spp. is found as a normal flora of skin, gastrointestinal tract and urogenital tract, in favourable conditions it can colonize and invade deeper into the host tissue. The predisposing factors of VVC are pregnancy, diabetes, use of steroids, repeated or prolonged use of antibiotics, hormonal replacement therapy (HRT), vaginal douching, oral-contraceptives and HIV/AIDS [1, 6, 8]. VVC is characterized by curd like vaginal discharge, vulval itching and erythema [1, 2, 7]. Furthermore, vulvovaginitis can be caused by Trichomonas vaginalis. It usually presents in females with frothy, foul smelling, green or yellow colour vaginal discharge, pruritus, dysuria, dyspareunia, or lower abdominal pain while 30% females are asymptomatic [6, 2, 9]. Patients of vulvovaginitis are at higher risk of acquiring sexually transmitted infections (STI) as pathological changes in vulvovaginitis facilitates other vaginal pathogens to gain access to the upper genital tract [2, 4, 9]. Resolution of vulvovaginitis is usually uncomplicated when diagnosed timely and treated appropriately but in case of delay in diagnosis and treatment it can pose a high risk of pelvic inflammatory disease (PID) and tubal infertility [2, 8, 9, 11]. In pregnant females, it can lead to preterm labour, premature rupture of membranes or postpartum endometritis [6, 11, 12].
In India, despite its common presence, vulvovaginitis is under-reported as females are hesitant to come forward with vaginal complaints due to its intimate nature. Unrecognized cases or delayed treatment of vaginitis can adversely affect physical and mental well-being of patients as well as initiation of treatment without knowing the etiology can lead to antimicrobial resistance in the community.
Thus, the present study was carried out to determine the etiological agents causing vulvovaginitis and associated risk factors among women attending a tertiary care hospital.
Materials and methods
This cross-sectional study was conducted at Rohilkhand medical college and hospital, a tertiary care hospital in Bareilly, over a period of one year from July 2023 to June 2024 after approval from the institutional ethics committee. Sample size was calculated as 100, assuming prevalence as 45% [13] using the formula 4*p*q/d2. Here, p is the prevalence, q is calculated as 100 − p, and d is taken as 10%. A total of 150 patients were included in the study.
Two vaginal swabs each from 150 patients of reproductive age group who presented with complaints of vulvovaginitis, were collected after informed consent and received in the department of Microbiology. Detailed history was recorded about patient’s demography, risk factors and clinical features. Women who were pregnant, menstruating, post-menopausal or who had taken vaginal or oral antibiotics in last 2 weeks were excluded from the study. One vaginal swab was subjected to Gram stain, wet mount, KOH mount examination and whiff test for the presence of clue cells, budding yeast-like cells and motile Trichomonads. Nugent’s scoring was performed in cases of bacterial vaginosis [14]. The second vaginal swab was inoculated on Sabouraud’s dextrose agar and incubated at 37 °C for 48 hours. The growth obtained on culture media was processed according to standard microbiological procedures [15]. The data was recorded and analyzed using SPSS version 23.0. Descriptive statistics were used to calculate the frequencies, percentages, mean and standard deviation (SD). The associations were analyzed using Chi square test of independence. A p- value of <0.05 was considered as statistically significant at a 95% confidence interval.
Results
Demographic data, risk factors, clinical signs and symptoms of all 150 patients were recorded and analysed. We have observed that majority of study population belonged to 21-40 years age group (74.66 %) followed by >40 years age group (18.6%). The median age of our study population was 32 years (Figure 1).
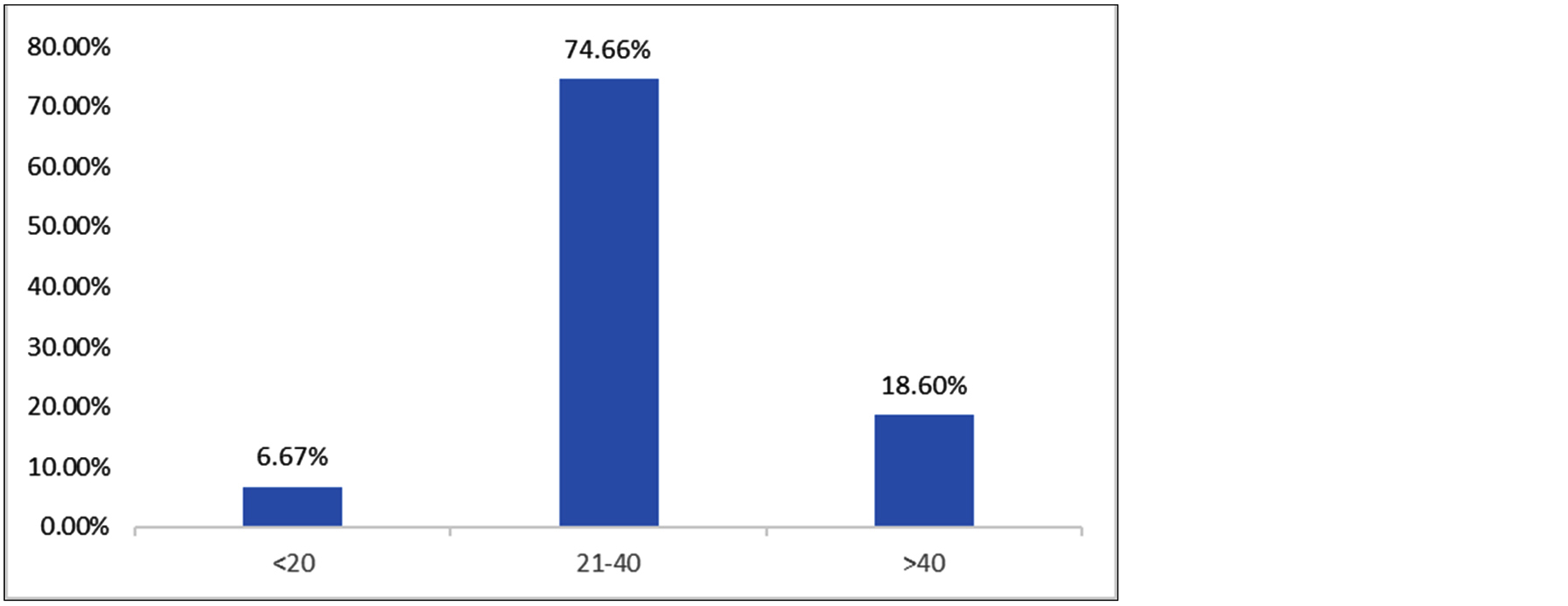
Figure 1: Age wise distribution of the study participants.
In our study, infective etiology could be established in 48% patients of vulvovaginitis. BV (29.3%) was found as the most common cause of vaginitis followed by VVC (18%) and trichomoniasis (0.6%). Mixed infection of BV and VVC was demonstrated in 3.3% cases. (Figure 2).
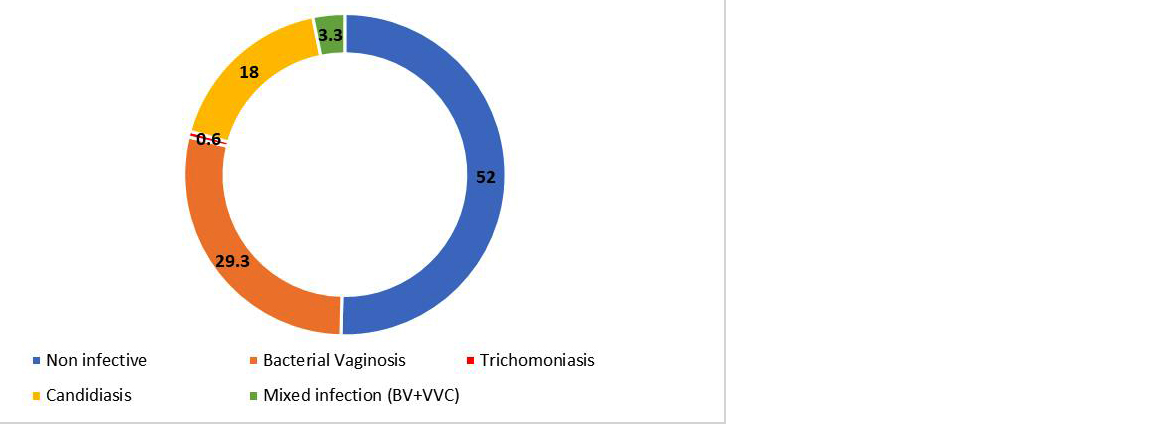
Figure 2: Distribution of etiology of vulvovaginitis in study participants (%).
Vaginal discharge was found as a presenting complaint in all the study participants. Various characteristics of discharge like duration, colour, consistency, quantity, odour and pH were recorded in association with etiology. The other common symptoms observed were vulval itching (63.3%), dyspareunia (31.3%), pain in abdomen (18%) and dysuria (14.6%) (Table 1).
Table 1: Association between clinical symptoms and microbial diagnosis.
|
Symptom
|
BV (44)
|
VVC (27)
|
TV (1)
|
|
1. Discharge characteristics (100%)
|
|
(a) Colour
|
|
White
Curdy white
Greenish
|
41 (93.2%)
3 (6.8%)
0
|
3 (11.1%)
24 (88.9%)
0
|
0
0
1 (100%)
|
|
(b) Consistency
|
|
Thick
Thin
|
3 (6.8%)
41 (93.2%)
|
25 (92.6%)
2 (7.4%)
|
1 (100%)
0
|
|
(c) pH
|
|
<5
>5
|
2 (4.5%)
42 (95.5%)
|
24 (88.9%)
3 (11.1%)
|
0
1 (100%)
|
|
(d) Malodour
|
12 (27%)
|
18 (66.7%)
|
1 (100%)
|
|
(e) Quantity
|
|
Scanty
Copious
|
16 (36.3%)
28 (63.7%)
|
18 (66.7%)
9 (33.3%)
|
0
1 (100%)
|
|
(f) Duration
|
|
<15 d
>15 d
|
30 (68.2%)
14 (31.8%)
|
23 (85.2%)
4 (14.8%)
|
1 (100%)
0
|
|
2. Vulval itching (63.3%)
|
28 (63.7%)
|
25 (92.6%)
|
1 (100%)
|
|
3. Pain abdomen (18%)
|
2 (4.5%)
|
1 (3.7%)
|
0
|
|
4. Dysuria (14.6%)
|
2 (4.5%)
|
4 (14.8%)
|
0
|
|
5. Dyspareunia (31.3%)
|
3 (6.8%)
|
14 (51.8%)
|
0
|
In our study population, various risk factors were evaluated and it was observed that substantial number of enrolled patients had history of use of contraceptives (30%), unhygienic practices (17.3%), previous intake of antibiotics more than 2 weeks prior (8%), previous episodes of STIs (6.6%), diabetes (5.3%) and habit of douching (4.6%). The other associated risk factors were menstrual irregularities (10%) and multiple sexual partners (3.3%) (Table 2).
A higher prevalence of BV was found in association with reproductive age group and unhygienic practices while recent intake of antibiotics appeared to decrease the prevalence of BV. Advanced age, diabetes and recent use of antibiotics were found as important risk factors for higher occurrence of VVC (Table 2).
Table 2: Distribution of risk factors of vulvovaginitis associated with various etiologies.
|
Risk factor
|
N (%)
|
BV
|
VVC
|
TV
|
|
BV (44)
|
No BV (106)
|
P value
|
VVC
(27)
|
No VVC
(123)
|
P value
|
TV
(1)
|
No TV
(149)
|
P value
|
|
Age
|
<40 yrs
|
122(81.3%)
|
37(84%)
|
85(80.1%)
|
0.57
|
20(74%)
|
102(82.9%)
|
0.28
|
1(100%)
|
121(81.2%)
|
0.42
|
|
>40 yrs
|
28(18.6%)
|
7 (15.9%)
|
21(19.8%)
|
7(25.9%)
|
21 (17%)
|
0
|
28(18.8%)
|
|
Unhygienic practices
|
26(17.3%)
|
12(27.2%)
|
14 (13.2%)
|
0.04
|
7 (25.9%)
|
19 (15.4%)
|
0.19
|
0
|
26(17.4%)
|
0.39
|
|
Douching
|
7 (4.6%)
|
3 (6.8%)
|
4 (3.7%)
|
0.42
|
0
|
7 (5.6%)
|
0.44
|
0
|
7 (4.6%)
|
0.03
|
|
Menstrual irregularities
|
15 (10%)
|
1 (2.2%)
|
14 (13.2%)
|
0.04
|
0
|
15 (12.1%)
|
0.12
|
0
|
15(10%)
|
0.18
|
|
Multiple sexual partners
|
5 (3.3%)
|
0
|
5 (4.7%)
|
0.33
|
1(3.7%)
|
4(3.2%)
|
0.63
|
1(100%)
|
4(2.6%)
|
0.009
|
|
Old h/o of STD
|
10 (6.6%)
|
0
|
10 (9.4%)
|
0.08
|
1 (3.7%)
|
9 (7.3%)
|
0.49
|
0
|
10(6.7%)
|
0.08
|
|
Diabetes
|
8 (5.3%)
|
0
|
8 (7.5%)
|
0.14
|
4(14.8%)
|
4 (3.2%)
|
0.01
|
0
|
8 (5.3%)
|
0.04
|
|
h/o antibiotic administration (>2 weeks)
|
12(8%)
|
0
|
12 (11.3%)
|
0.04
|
5(18.5%)
|
7(5.6%)
|
0.03
|
0
|
12(8%)
|
0.12
|
|
Contraceptive practices
|
45(30%)
|
8 (18.1%)
|
37(34.9%)
|
0.29
|
13(48%)
|
32(26%)
|
0.02
|
0
|
45(30.2%)
|
0.66
|
Discussion
Vaginal discharge is a common complaint among women of reproductive age group. The discharge can be either physiological or pathological. Various pathogens like bacteria, fungi, protozoa and viruses can be associated with vaginal discharge. In our study, the average age (SD) of the study population was 31.9 (7.8) years. More than two third (74.66%) of the study participants belonged to 21-40 years age group. Similar demographic results were found in studies conducted by Anandan et al, Shanmugam et al, John et al, and Gopal et al [6, 7, 10, 16]. This is probably because the women of this age group are in reproductive age and are sexually active. Among 150 study participants with complaints of vaginitis, vaginal discharge was the universal symptom present in all the patients. However, microbiological diagnosis could be established only in 72 (48%) patients. Similar prevalence of infective vaginitis was reported by Tankhiwale et al (44.9%) [13]. Studies done by Anandan V et al and Shanmugam et al reported a higher proportion of infective etiology in patients of vulvovaginitis [6, 7]. Varying prevalence rates of infective vulvovaginitis in various studies might be due to difference in demographic factors, education, socioeconomic status, diagnostic techniques and geographical location. Apart from vaginal discharge, other common symptoms were vulval itching (63.3%) and dyspareunia (31.3%). Similar clinical spectrum was depicted by John et al and Kiran et al in their studies [10, 17].
Bacterial vaginosis (44/72 cases) accounted for approximately half of microbiologically confirmed cases of vulvovaginitis in our study. Kiran et al (22%) and Gopal et al (32%) reported similar occurrence of BV while Shanmugam et al (42.3%) and Anandan et al (38%) reported higher occurrence of BV in their studies [6, 7, 16, 17]. Occurrence rate of BV is found to vary from 19%- 42% in various studies but most of the studies demonstrated BV as the predominant etiology of vulvovaginitis [6, 7, 16]. Among 44 patients diagnosed with BV, the predominant symptom was the presence of a thin, white vaginal discharge which was observed in 93.2% of cases. Most BV cases had a vaginal pH >5 (95.5%), which aligns with classical diagnostic criteria for BV. Malodorous discharge was reported in 27.3% of BV cases. Vulval itching was present in 63.6% of BV cases. These findings were consistent with previous studies like Anandan et al and Kiran et al noted that BV is frequently characterized by a thin, homogeneous discharge and elevated vaginal pH, often accompanied by a fishy odor due to amines [6, 17]. In the present study, a statistically significant association was observed between unhygienic practices and BV (P<0.05). This observation aligns with various studies that highlight poor genital hygiene as a risk factor for BV [12, 17, 18]. World health organization (WHO) also recognizes unhygienic practices as a modifiable risk factor for BV and recommends good personal hygiene such as proper washing, wiping front-to-back and avoiding soiled clothing as a preventive strategy for BV [19]. Regular menstrual cycles may cause predictable estrogen fluctuations resulting in instability of vaginal microbiota and rise in vaginal pH, hence facilitating BV pathogenesis [8, 20]. The present study showed low proportion of menstrual irregularities in BV patients (2.2%), compared to a significantly higher proportion in non-BV patients, suggesting a inverse association between menstrual irregularities and BV. While statistically significant, a larger sample size would strengthen this association. This study showed negative association between history of previous antibiotics intake and BV (p<0.05). Often, antibiotics are considered as potential risk factor for vaginal dysbiosis by disrupting lactobacilli dominant vaginal flora. However, some previous studies also demonstrated that recent treatment by narrow spectrum antibiotics like metronidazole and clindamycin can reduce current colonization by BV-associated bacteria [21, 22].
VVC was elicited as the next common cause of abnormal vaginal discharge in 27 (18%) study participants which is in concordance with the study results of Anandan V et al (20%) and Shanmugam et al (19.4%) [6, 7]. In contrast, John N et al and Kiran CK et al reported VVC as the most common cause of vulvovaginitis [10, 17]. Of the 27 VVC cases, 88.9% exhibited curdy white discharge and 92.6% reported vulval itching, symptoms which are commonly associated with candidal infections. The vaginal pH in most VVC patients was <5 (88.9%), consistent with the acidic environment conducive to Candida growth [23]. Dyspareunia was noted in 51.9% of cases, indicating a significant impact on sexual health. The strong correlation between symptoms such as vulval itching and dyspareunia in VVC is echoed in research by Kiran et al and Richter et al, which found high rates of discomfort and sexual dysfunction in candidiasis patients [18, 24]. A statistically significant association between diabetes mellitus and VVC was observed in this study (14.8% of VVC patients vs 3.2% of non-VVC, p< 0.05). Diabetes is a well-documented risk factor for recurrent and severe candidal infections as hyperglycaemia creates a favourable environment for candidal growth which promotes fungal adherence and proliferation [8]. Similarly, previous studies have shown that women with diabetes are more likely to develop symptomatic VVC and to experience recurrent episodes [7, 25]. We have observed a significant association between prior antibiotic use and increased incidence of VVC (p <0.05) which is well-supported by existing literature. Broad-spectrum antibiotics are known to disrupt the normal vaginal microbiota that creates an opportunity for commensal Candida to proliferate [8, 25, 26].
Additionally, we have also observed a significant association between contraceptive use and VVC in the present study (48% in VVC group vs 26% in non-VVC group, p<0.05) which is consistent with findings reported in various studies [9, 26, 27]. Hormonal contraceptives can predispose women to VVC by disrupting the protective vaginal microbiota and promoting glycogen deposition in vaginal epithelial cells, thereby creating favourable conditions for candidal proliferation [8, 26, 28].
In our study, only a single case of TV (0.6%) was found, showing greenish, thick, and copious discharge with elevated vaginal pH and associated malodour, which is consistent with classical presentation of trichomoniasis in the literature [1, 26]. Although the study showed significant association between risk factors like douching, multiple sexual partners, diabetes (p <0.05) and trichomoniasis, presence of a single case of TV limits statistical significance.
The main limitation in our study was that in approximately 50% of the participants with symptoms of vaginitis, a microbiological diagnosis could not be made. This indicates that other etiologies including aerobic vaginitis, chlamydia and other sexually transmitted infections might be involved, which were not investigated in the study.
Conclusion
This study showed a significant burden of vulvovaginitis among women of reproductive age group. BV was found to be the most prevalent etiology, followed by VVC and trichomoniasis. Vaginal discharge was a universal symptom among study participants, with vulval itching and dyspareunia also commonly reported. Microbiological confirmation of infection was possible in nearly half the cases, underscoring the importance of laboratory diagnosis in guiding appropriate treatment. Risk factors such as unhygienic practices, diabetes mellitus, previous antibiotic use, and hormonal contraceptive use were found to be significantly associated with vulvovaginitis. The study emphasizes the need for awareness, timely diagnosis, and targeted management of vulvovaginitis to prevent complications such as recurrent infections, pelvic inflammatory disease, and negative impacts on reproductive health. Strengthening public health education and promoting genital hygiene can play a pivotal role in reducing the incidence of these infections.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020; 19:1–19.
[2] Quan M. Vaginitis: diagnosis and management. Postgrad Med. 2010; 122:117–127.
[3] Martin DH. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci. 2012; 343:2–9.
[4] Leclair C, Stenson A. Common causes of vaginitis. JAMA. 2022; 327:2238–2239.
[5] Masand DL, Patel J, Gupta S. Utility of microbiological profile of symptomatic vaginal discharge in rural women of reproductive age group. J Clin Diagn Res. 2015; 9:QC04–QC07.
[6] Anandan V, Kayalvizhi A, Vijayabhaskar C, Sobimeena RM. A study on vaginal discharge in females attending sexually transmitted diseases outpatient department. Int J Res Dermatol. 2022; 8:314–319.
[7] Shanmugam NP, Balasundharam A, Thomas IN, Radhakrishnan A, James JJ. A cross-sectional clinical investigation of organisms causing vaginal discharge in patients in rural Tamil Nadu, India. Cureus. 2023; 15:e33979.
[8] Sobel JD, Vempati YS. Bacterial Vaginosis and Vulvovaginal Candidiasis pathophysiologic interrelationship. Microorganisms. 2024; 12:108.
[9] Huang SH, Hsu HC, Lee TF, Fan HM, Tseng CW, et al. Prevalence, associated factors, and appropriateness of empirical treatment of trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis among women with vaginitis. Microbiol Spectr. 2023; 11:e0016123.
[10] John N, Rahima S, Raji TK, Santhosh P, Kidangazhiathmana A, et al. Clinicoetiological study on vaginal discharge among sexually active women attending a tertiary center in North Kerala, India. Indian J Sex Transm Dis AIDS. 2023; 44:1–5.
[11] Rajalakshmi R, Kalaivani S. Prevalence of asymptomatic infections in sexually transmitted diseases attendees diagnosed with bacterial vaginosis, vaginal candidiasis, and trichomoniasis. Indian J Sex Transm Dis AIDS. 2016; 37:139–142.
[12] Ranjit E, Raghubanshi BR, Maskey S, Parajuli P. Prevalence of bacterial vaginosis and its association with risk factors among nonpregnant women: A hospital-based study. Int J Microbiol. 2018; 2018:8349601.
[13] Tankhiwale SS, Sharma MK, Surpam RB, Fussey SS. Etiological study of vaginal discharge syndrome in RTI clinic attendees in a tertiary care hospital. New Indian J OBGYN. 2017; 4:50–53.
[14] Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991; 29:297–301.
[15] Collee JG, Fraser AG, Marmion BP, Simmons A. Mackie & McCartney Practical Medical Microbiology. 14th ed. Edinburgh: Churchill Livingstone; 1996.
[16] Gopal V, Gopal R, Rupavani, Mangaiyarkarasi T. Clinico-etiological study of vaginal discharge in adult women: A hospital-based study. Indian J Microbiol Res. 2018; 5:535–537.
[17] Kiran CK, Kandati J, Ponugoti M. Etiologic characterization of vulvovaginitis among females attending a tertiary care hospital: a one-year study. Int J Reprod Contracept Obstet Gynecol. 2017; 6:2246–2251.
[18] Samantaray SR, Parida S, Mohapatra I. Connecting the dots: Exploring the relationship between menstrual hygiene and bacterial vaginosis in eastern India. J Family Med Prim Care. 2024; 13:4451–4456.
[19] World Health Organization. Guidelines for the management of symptomatic sexually transmitted infections. Geneva: World Health Organization; 2021.
[20] Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014; 21:450–458.
[21] Khedkar R, Pajai S. Bacterial vaginosis: A comprehensive narrative on the etiology, clinical features, and management approach. Cureus. 2022; 14:e31314.
[22] Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007; 44:S102–S110.
[23] Sobel JD. Vulvovaginal candidosis. Lancet. 2007; 369:1961–1971.
[24] Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, et al. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005; 43:2155–2162.
[25] Irene VR, Sajeeth CI, Karthikeyan V, Sabitha J. Assessment of risk factors for developing vulvovaginal candidiasis among women at various age groups. Biosci Biotechnol Res Asia. 2023; 20:359–365.
[26] Dalby J, Stoner BP. Sexually Transmitted Infections: Updates From the 2021 CDC Guidelines. Am Fam Physician. 2022; 105:514–520.
[27] Salvi M. Prevalence of vulvovaginal candidiasis in females in the reproductive age group. Int J Reprod Contracept Obstet Gynecol. 2019; 8:647–651.
[28] Sustr V, Foessleitner P, Kiss H, Farr A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J Fungi. 2020; 6:267.