Full Text
Introduction
Lymphoma is the third most common pediatric neoplasm, trailing only leukemia and brain tumors, constituting 10–15% of childhood cancers, making lymphoma a significant concern in pediatric oncology within the country [1, 2]. Pediatric lymphomas are a diverse group of lymphoproliferative malignancies, each with unique patterns of behavior and varying responses to treatment. The two main categories are non-Hodgkin lymphoma and Hodgkin lymphoma. The common subtypes among pediatric NHL are T lymphoblastic lymphoma (T-LBL), Burkitt lymphoma (BL) and B lymphoblastic lymphoma (B-LBL), each characterized by unique cellular morphology, genetic aberrations and clinical behaviors. This pathological diversity underscores the intricate nature of NHL, necessitating tailored diagnostic and therapeutic strategies for optimal patient management [3, 4]. Pediatric NHL generally behaves in a high-grade manner and commonly presents with extranodal involvement than adults [5].
Nodular sclerosis classic Hodgkin lymphoma (NSCHL) represents the predominant subtype, followed by mixed cellularity classic Hodgkin lymphoma (CHL), particularly prevalent among the older pediatric population. Encouragingly, pediatric HL demonstrates an impressive cure rate exceeding 90%, chiefly attributable to the efficacious synergy of chemotherapy and low-dose radiation therapy [6, 7].
The objectives of the study were to determine the incidence and subtypes of pediatric lymphoma, as well as their prognostic parameters. We analyzed the distribution of nodal and extranodal lymphoma in the pediatric population.
Materials and methods
This is a retrospective observational study conducted from January 2020 to July 2023 in the Department of Oncopathology at Gujarat Cancer and Research Institute, Ahmedabad, the state cancer institute encompassing 126 patients including 68 review cases with age spectrum of 0 to 18 years after obtaining the ethical committee approval.
All lymphoma cases with the help of histomorphology and IHC were enrolled. Individuals with a previous history of malignancy, chemotherapy or radiotherapy as well as cases with insufficient biopsy materials were excluded from the study.
All the biopsies were fixed in 10% neutral buffered formalin, paraffin embedded and sectioned at 3 - 4 micron for hematoxylin and eosin (H & E) staining. Wright-stained bone marrow aspiration (BMA) smears and Giemsa stained cerebrospinal fluid (CSF) cytospins, were utilized for prognostic assessment at the time of diagnosis. All cases were diagnosed with the help of histomorphological features such as architecture disruption, cell patterns, cell size, nucleus, nucleolus, cytoplasm, reactive cells, fibrosis, necrosis and mitosis. Each diagnosis was corroborated through adherence to morphological and IHC criteria outlined in the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours [7].
Lymphoma classification was determined using a panel of monoclonal antibodies in IHC, which included LCA(PD7/26/16+2B11), CD20(L26), CD3 (F7.2.38), CD2(AB75), CD4(SP35), CD5(SP19), CD7(CBC.37),CD8(SP57), CD10(SP6), BCL2(100/D5), BCL6(BL6.02), MUM1(SP114), C-MYC(9E10), TdT(EP266), CD34 (QBEnd/10), CD79a(SP18), CD99(MIC-2)(H0361.1), MPO(Polyclonal), CD19(EP169), ALK1(5A4), EMA(E29), PAX5 (DAK-Pax5), CD15(Cab-3), CD30(Ber-H2), BOB1(SP92), OCT2(EP115), Fascin(BSB36), CD56 (123C3.D5), CD43(L60), CD38 (SP149), CD138 (MI15), CD21(1F8), CD23(MRQ-57) and Ki-67(MIB1). IHC was done on Ventana Benchmark XT autoimmunostainer using ventana reagent.
Results
Over the course of three and a half years, our study enrolled a total of 126 pediatric lymphoma patients. In our study, the median age at the time of diagnosis was 10.4 years, ranging from 11 months to 18 years. The age group with the highest frequency (32.6%) was between 5 to 9 years, with the 10 to 14 years group following closely (31.7%), while the lowest incidence (11.1%) was observed in the 0 to 4 years age group. 92 cases (73%) observed in males and 34 cases (27%) in females. This distribution reflects a male-to-female ratio of 2.7:1.
Illuminating the spectrum of non-Hodgkin lymphoma and Hodgkin lymphoma in pediatric patients
Of the total 126 cases, NHL constituted 67 cases (53.2%), while HL accounted for 59 cases (46.8%). This detailed differentiation highlights the prevalence of NHL over HL within the pediatric population.
Categorization of pediatric non-Hodgkin lymphoma based on cellular lineage
In a study of 67 pediatric NHL cases using IHC, 50.7% (n = 34) exhibited T-cell lineage markers, while 49.3% (n = 33) showed B-cell lineage markers. This distribution underscores the molecular heterogeneity of pediatric NHL and underlines the importance of precise diagnostic approaches.
Pediatric non-Hodgkin lymphoma
Total 67 pediatric non-Hodgkin lymphoma cases are detailed in Table 1.
Table 1: Clinicopathological characteristics of pediatric non-Hodgkin lymphoma.
|
Clinicopathological characteristics
|
Number of pediatric NHL patients
(n = 67)
|
Percentage
(%)
|
|
Gender
|
|
|
Male
|
51
|
76.1
|
|
|
Female
|
16
|
23.9
|
|
LDH
|
|
|
< 500
|
22
|
32.8
|
|
|
>= 500
|
40
|
67.2
|
|
Anatomical biopsy site
|
|
|
Peripheral lymph nodes
|
20
|
29.8
|
|
|
Abdominal region
|
18
|
26.9
|
|
|
Thoracic cavity
|
17
|
25.4
|
|
|
Head and neck
|
9
|
13.4
|
|
|
Pelvic region
|
2
|
3.0
|
|
|
Central nervous system (CNS)
|
1
|
1.5
|
|
Bone marrow involvement
|
14
|
20.9
|
|
Cerebrospinal fluid involvement
|
6
|
8.9
|
|
Histomorphological with IHC subtypes
|
|
|
T lymphoblastic lymphoma (T-LBL)
|
31
|
46.3
|
|
|
Burkitt lymphoma (BL)
|
17
|
25.4
|
|
|
B lymphoblastic lymphoma (B-LBL)
|
9
|
13.3
|
|
|
Diffuse large B cell lymphoma, non - germinal center B cell subtype (DLBCL, non-GCB)
|
3
|
4.5
|
|
|
ALK+ anaplastic large cell lymphoma (ALK+ALCL)
|
3
|
4.5
|
|
|
Extranodal marginal zone lymphoma (EMZL)
|
2
|
3.0
|
|
|
Primary mediastinal large B cell lymphoma (PMBCL)
|
2
|
3.0
|
T lymphoblastic lymphoma
In 46.3% (n=31) cases of T-LBL, males accounted for 80% and females for 20%, with median age of onset at 11 years. Notably, the most frequent sites of occurrence were lymph nodes and the thoracic cavity (mediastinum and pleura). Histomorphology showed diffuse infiltration by medium-sized atypical lymphoid cells with high N:C ratio, finely dispersed chromatin, small nucleoli, irregular nuclear contour and scanty cytoplasm with multiple mitotic figures. IHC profile revealed positivity for CD3 and TdT in all cases and for CD5 and CD7 in 75-80% of cases. The Ki67 proliferation index ranged from 85% to 98% (Figure 1).
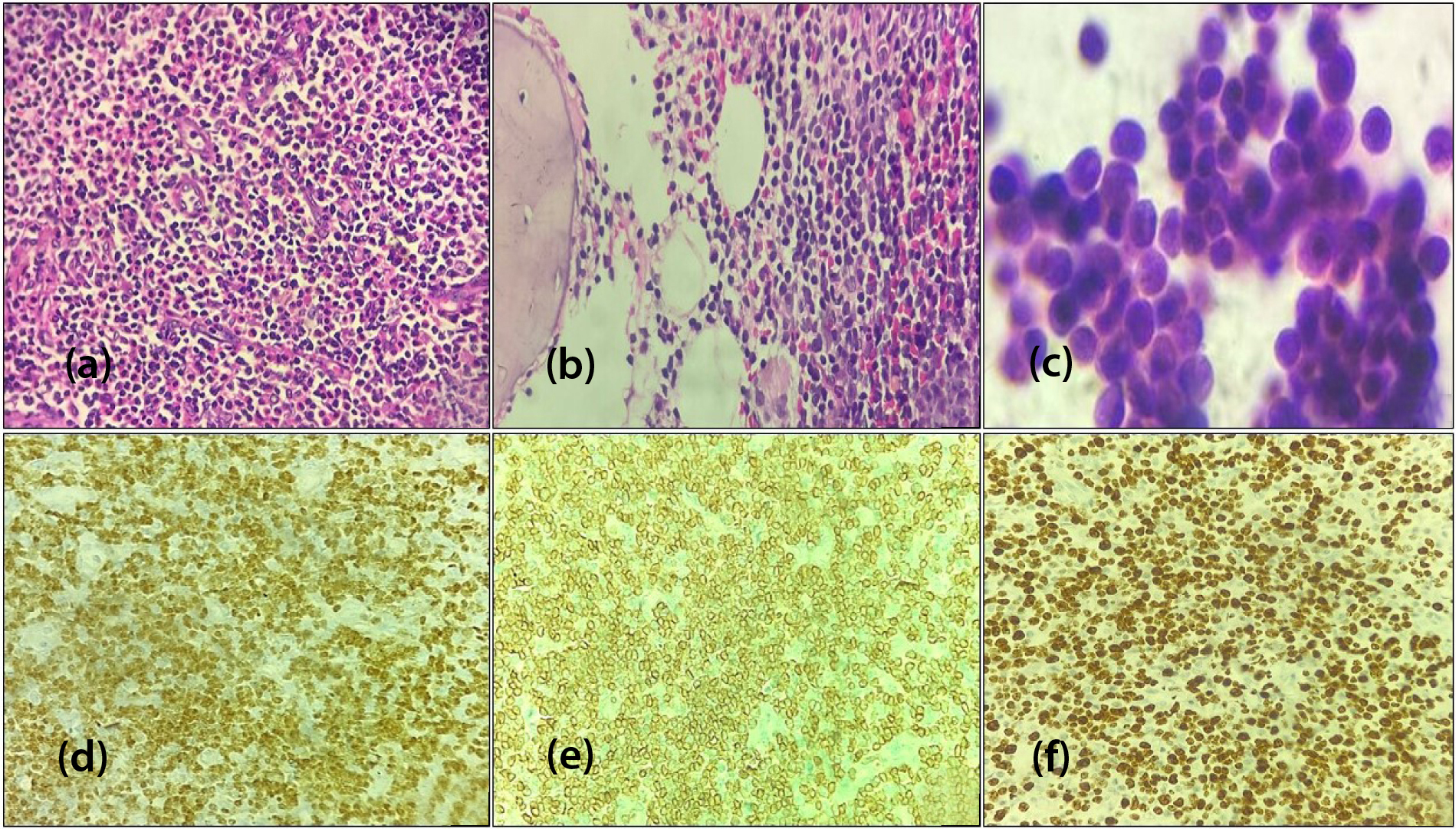
Figure 1: T lymphoblastic lymphoma, (a) Mediastinal biopsy [H&E, x400], (b) Bone marrow biopsy [H&E, x400] (c) CSF cytology smear showing infiltration by lymphoblasts [Giemsa stain, x400], (d) TdT nuclear positive [IHC, x400], (e) CD3 membranous positive [IHC, x400] (f) High Ki67 index (90-95%) [IHC, x400].
Burkitt lymphoma
Of the total cases, 25.4% (n=17) were diagnosed as BL. The abdominal region and intestine emerged as the primary site of occurrence. Morphologically, characterized by monotonous medium-sized lymphoid cells with round nuclei, squared nuclear membranes, coarse chromatin, multiple nucleoli and basophilic cytoplasm. The background featured numerous tingible body macrophages, creating a starry-sky appearance, along with abundant mitoses and apoptotic bodies. In all 5 cases, BMA highlighted cytoplasmic vacuolation in lymphoid cells. IHC consistently revealed positivity for CD10, BCL6, C-MYC and CD20 while BCL2, CD3 and TdT were negative. The Ki67 proliferation index was nearly 98 to 100% (Figure 2).
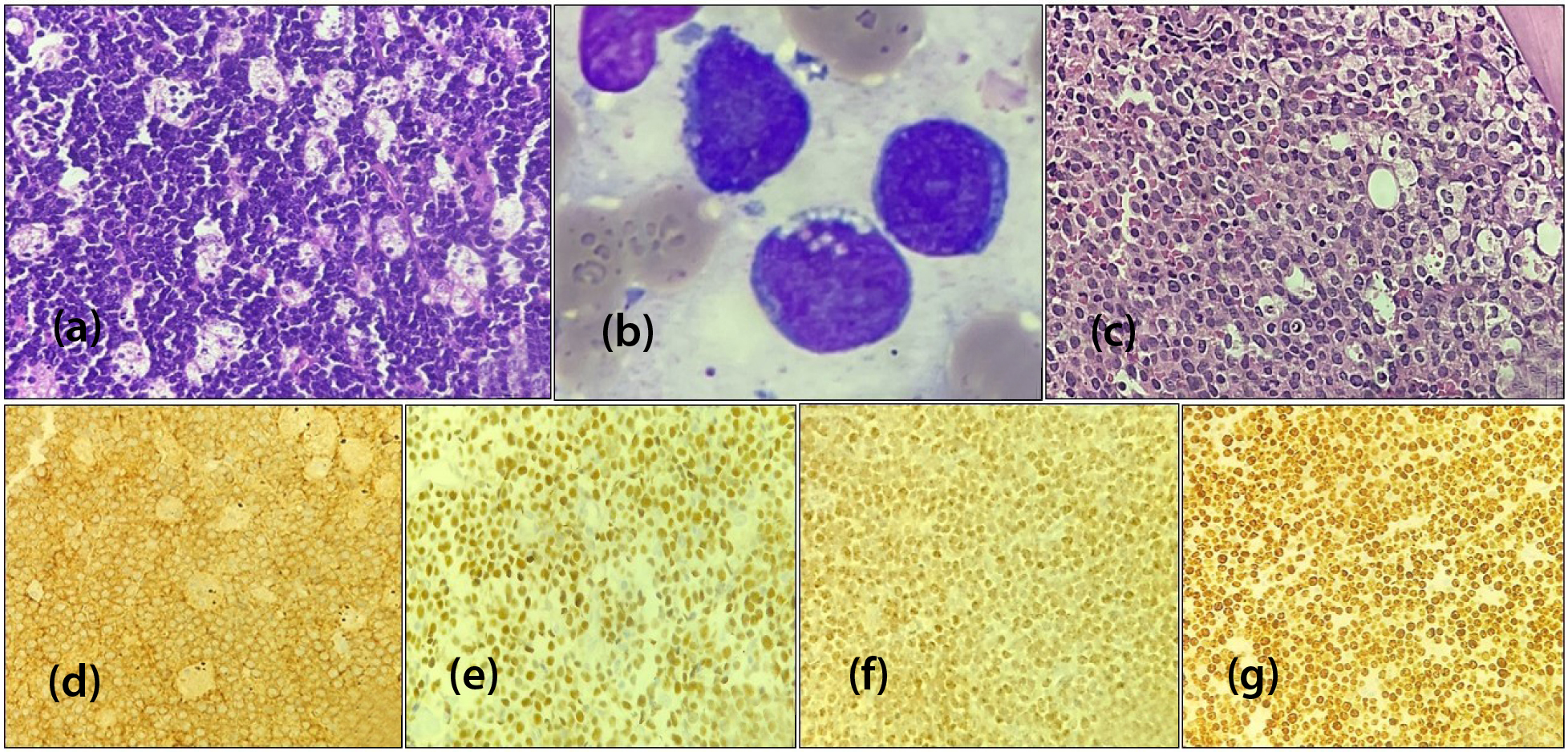
Figure 2: Burkitt lymphoma, (a) Ileum biopsy [H&E,x400], (b) Bone marrow aspiration smear [Wright stain,x1000], (c) Bone marrow biopsy [H&E,x400], (d) CD10 membranous positive [IHC,x400], (e) BCL6 nuclear positive [IHC,x400], (f) C-MYC nuclear positive [IHC,x400] (g) High Ki67 index (98-100%) [IHC,x400].
B lymphoblastic lymphoma
Nine cases (13.3%) of B - LBL were identified with most involving cervical lymphnodes, pelvic region and scalp. H & E examination showed uniform small to medium sized lymphoblasts with scanty cytoplasm, round nuclei, condensed chromatin, high N:C ratio and characterized by positivity for B-cell markers along with CD34, HLA-DR and negativity for T-cell markers on IHC.
Diffuse large B cell lymphoma
We reported 4.5% (n=3) of DLBCL were histologically characterized by a diffuse proliferation of large, atypical lymphoid cells with abundant cytoplasm, vesicular nuclei, prominent nucleoli, and a high mitotic rate. Pan B cell markers and MUM1 were positive, with CD10 and BCL6 negative on IHC in our findings, indicating a non-germinal center B cell (non-GCB) phenotype in all cases.
ALK positive anaplastic large cell lymphoma
In our study, 4.5 % (n=3) of ALCL displayed large, pleomorphic hallmark cells characterized by abundant eosinophilic cytoplasm, vesicular chromatin, prominent nucleoli and positive IHC staining for cytoplasmic granular ALK protein, CD30 and T cell markers.
Extranodal marginal zone lymphoma
We found 3% (n=2) of mucosa-associated lymphoid tissue (MALT) lymphoma with varied histology, including centrocyte-like cells, monocytoid cells, and scattered immunoblasts derived from small mature B cells. On IHC, CD19 and PAX5 were positive, while CD10, BCL6, cyclinD1 and T cell markers were negative.
Primary mediastinal large B-cell lymphoma
Rare subtype, 3% (n=2) of pediatric NHLs in our study, presented with anterior mediastinal mass and compartmentalizing fibrosis.
Pediatric Hodgkin lymphoma
The characteristics of 59 pediatric HL patients are summarized in Table 2 and Table 3.
Table 2: Clinical profiles of pediatric Hodgkin lymphoma.
|
Clinical profiles
|
Number of pediatric HL patients (n = 59)
|
Percentage
(%)
|
|
Gender
|
|
|
Male
|
39
|
66.1
|
|
|
Female
|
20
|
33.9
|
|
Age group (years)
|
|
|
0 – 4
|
3
|
5.1
|
|
|
5 – 9
|
17
|
28.8
|
|
|
10 – 14
|
20
|
33.9
|
|
|
15 – 18
|
19
|
32.2
|
|
ESR
|
|
|
< 20
|
35
|
59.3
|
|
|
>= 20
|
24
|
40.7
|
|
Bone marrow involvement
|
4
|
6.8
|
|
Nodal biopsy site
|
|
|
Cervical
|
34
|
57.6
|
|
|
Axillary
|
7
|
11.8
|
|
|
Supraclavicular
|
6
|
10.2
|
|
|
Inguinal
|
3
|
5.1
|
|
|
Mesenteric
|
2
|
3.4
|
|
Extranodal biopsy site
|
|
|
Mediastinum
|
6
|
10.2
|
|
|
Parotid gland
|
1
|
1.7
|
Table 3: Pathological landscape of pediatric Hodgkin lymphoma.
|
Histomorphological and IHC profiles
|
Number of pediatric HL patients (n = 59)
|
Percentage
(%)
|
|
Hodgkin lymphoma subtypes
|
|
|
Mixed cellularity CHL
|
33
|
56.0
|
|
|
Nodular sclerosis CHL
|
14
|
23.7
|
|
|
Lymphocyte rich CHL
|
5
|
8.5
|
|
|
Nodular lymphocyte predominant HL
|
4
|
6.8
|
|
|
Lymphocyte depleted CHL
|
3
|
5.0
|
|
Immunohistochemical marker positivity
|
|
|
CD30
|
54
|
91.5
|
|
|
CD15
|
50
|
84.7
|
|
|
PAX5 (weak)
|
55
|
93.2
|
|
|
OCT2
|
10
|
17.0
|
|
|
BOB1
|
7
|
11.9
|
Morphologically, CHL is characterized by mononuclear Hodgkin cells and multinucleated Reed-Sternberg (HRS) cells, amidst a polymorphous background of reactive immune cells. In our study, the standard IHC panel for confirming HL included CD30, CD15, PAX5, BOB1, OCT2, LCA, CD3 and CD20. Classical HRS cells typically showed strong membranous and Golgi CD30 positivity, membranous CD15 staining, weak nuclear PAX5 positivity, and were negative for LCA (Figure 3).
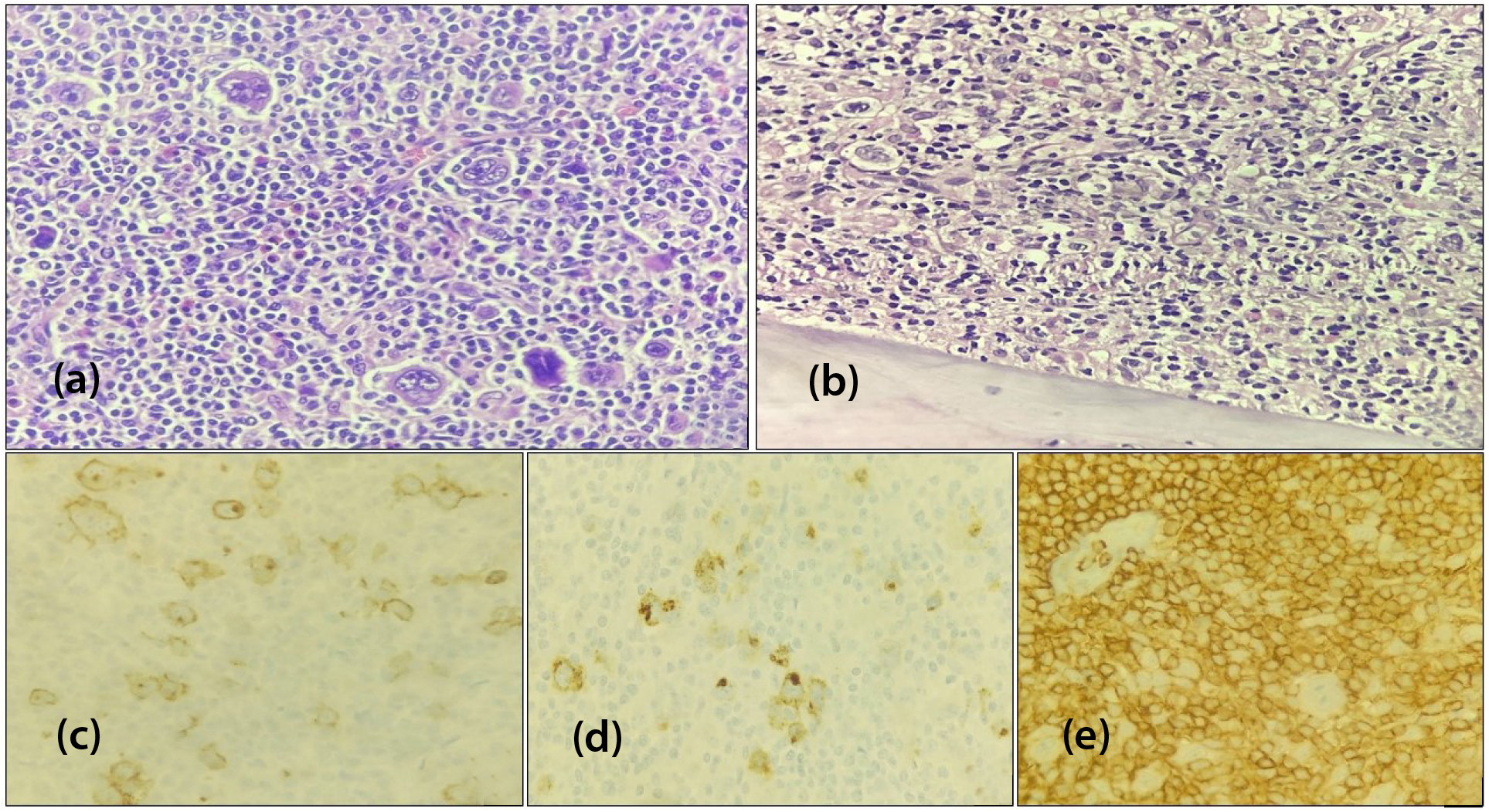
Figure 3: Hodgkin lymphoma, (a) Mixed cellularity CHL in cervical lymphnode biopsy [H&E, x400], (b) Bone marrow biopsy showing hypercellular marrow spaces comprising HRS cells [H&E, x200], (c) CD30 membranous and Golgi staining [IHC, x400] (d) CD15 membranous positive [IHC, x400], (e) LCA negative HRS cells [IHC, x400].
Discussion
Pediatric lymphoma constitute approximately 10-15 % of all childhood cancers and are broadly categorized into non-Hodgkin lymphoma and Hodgkin lymphoma. Through meticulous examination on aspects of pediatric lymphoma within the institution over the specified timeframe and aligning the findings with other similar studies, valuable insights can be contributed to the understanding and management of this disease in children. The distribution of histologic subtypes is reported differently across various regions worldwide [1, 2, 8].
In our study of 126 cases, the median age at the time of diagnosis was 10.4 years with children aged 0 to 4 years who showed the lowest incidence rate at 11.1% . Only 0.8% (n=1) NHL was discovered in infant age group which was comparable with Mann et al. [9], where 1% (n=20) NHL was found in infants, suggesting that NHL in infants is exceptionally rare.
Among 126 pediatric lymphoma cases, NHL comprised 53.2%, while HL accounted for 46.8% which was comparable to the study done in Brazil population by Gualco et al. [10], where NHL cases constituted 58% and HL cases were 42%.
The anatomical distribution at diagnosis holds prognostic significance since CNS involvement necessitates more intensive therapy [11]. In a recent study, pediatric NHL was observed in 70.1% (n=47) of extranodal sites, including the thoracic cavity, abdominal region, head and neck sites including oropharynx, thyroid and scalp, pelvic region, with rare occurrences also noted in the CNS and orbit, highlighting the diverse presentation.
In our study of western India, T lymphoblastic lymphoma comprised 46.3% (n=31) of pediatric NHL cases. Manipadam et al. [12] in south India, Lee S et al. [13] in Korea and Nakagawa et al. [14] in Japan reported T-LBL of 32.1%, 17.4% and 24%, respectively. CNS involvement by positive CSF cytology was observed in 12.9% (n=4) of the T-LBL group, similarly Huang et al. [15] reported 13.6% (n=14) with CNS involvement. These differences highlight the variability in T-LBL prevalence due to geographic factors and study populations. Moreover, CNS involvement is a poor prognostic factor for pediatric NHL.
Burkitt lymphoma was found to be 25.4% (n=17) in our exploration, primarily affecting the abdominal region and intestines, with 47 % (n=8) cases showing BM involvement. Suh et al. [16] reported 35.7% (n=268) BL cases, with 30.2% (n=81) exhibiting BM involvement. Notably, CNS and BM involvement were more commonly seen in patients with lymphoblastic and Burkitt lymphomas than in other subtypes.
In our study of nine reported cases of B-LBL, 5 cases were localized, and bone marrow involvement was observed in 4 patients. Conversely, in the study by Devine et al. [17] among 10 cases of B-LBL, 2 were localized and 8 were disseminated. Patients with localized disease tend to have a more optimistic prognosis.
In this study, 4.5% (n = 3) of DLBCL cases were classified as the non-GCB subtype using the Hans algorithm based on the IHC analysis of three markers (CD10, BCL6, MUM1), whereas Miles et al. [18], found that 74.7% (n = 59) were of the germinal center B cell (DLBCL - GCB) subtype, associated with a better outcome. This discrepancy is likely due to the limited number of patients in our DLBCL group.
ALCL is the most common pediatric peripheral T-cell lymphoma, constituting 4.5% (n=3) of pediatric NHL cases in our study, all of which were ALK-positive by IHC. This is similar to Dokmanovic et al. [19], who documented 8.8% (n=5) of ALCL cases in Serbia. Our three patients, followed up regularly, had a 100% event-free survival rate over 2.5 years. Pediatric ALCL cases are mostly ALK-positive and generally have a better prognosis than adults [20]. Low grade B-cell lymphomas are exceptionally uncommon in children as we observed only 3% (n=2) cases of EMZL affecting the intestine. In Gualco et al. [10] and Lee S et al. [13] EMZL constituted 0.6% and 1.6% of all NHL cases, respectively.
Two cases (3%) of PMBCL in pediatric patients showed compartmentalizing fibrosis and strong PAX5, with weak CD30 and CD23 positivity on IHC. This was similar to the 2.3% (n=11) of cases documented by Gualco et al [10].
In our analysis, 33.9% of HL cases were in the 10-to-14-year age group, and 32.2% were in the 15-to-18-year group, with a male-to-female ratio of 1.9:1. Nodal involvement was significantly detected in HL comprising 88.1% (n=52) with 10.2% mediastinum involvement in the NSCHL subtype. Histologically, mixed cellularity CHL was the most common subtype, accounting for 56% of cases, followed by NSCHL at 23.7%. This is similar to Sherief et al. [8], where mixed cellularity CHL was 50.8% and nodular sclerosis was 28.9%. Faizan et al. [21] also reported mixed cellularity as the most common subtype in pediatric patients. In contrast, Ali et al. [22] and Lee S et al. [13] observed that NSCHL was more prevalent than mixed cellularity CHL. Bone marrow involvement in HL is uncommon, yet its presence suggests stage IV disease and is associated with a worse prognosis. In our study 6.8% HL cases were presented with BM involvement at the time of diagnosis. Similarly Radhakrishnan et al. [23] reported as 3% of HL cases with BM involvement as primary presentation.
The regional distribution and histomorphology subtypes of pediatric lymphoma underscore the needs for careful differential diagnosis and emphasize the importance of further research in the pediatric population. We thus highlighted the diagnostic workflow approach for pediatric lymphoma (Figure 4).
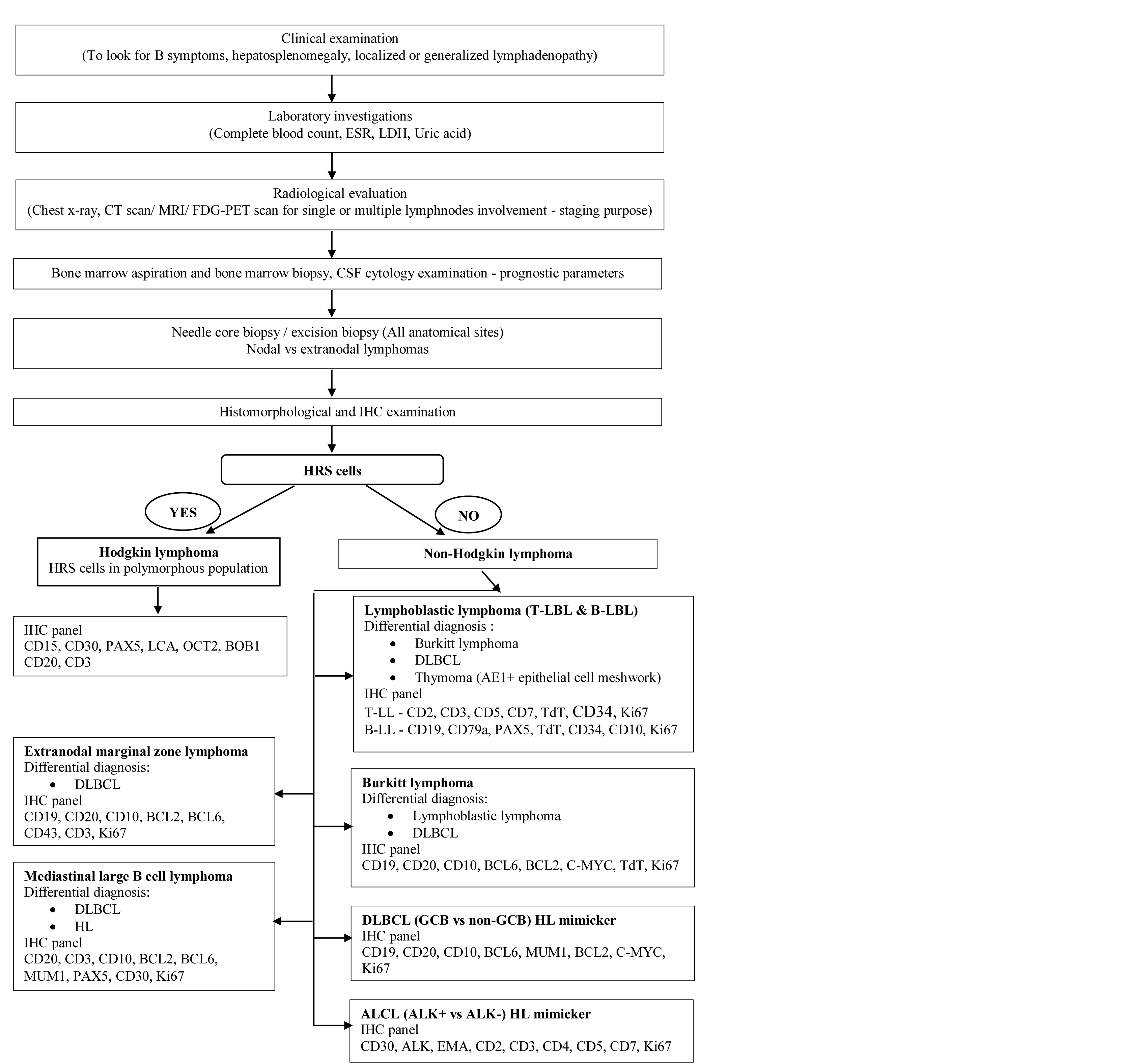
Figure 4: Diagnostic workflow approach for pediatric lymphoma.
Limitations: The study is limited by the lack of Ebstein-Barr encoding region in situ hybridization (EBER ISH) analysis and detailed genomic profiling which could provide deeper insights into genetic drivers of pediatric lymphomagenesis.
Conclusion
This paper provides an overview of the current landscape for pediatric lymphoma patients in a state cancer institute. Detailed morphological evaluation and a focused IHC panel are crucial for accurately subclassifying both nodal and extranodal lymphoma. Recent advancements in the field of pediatric lymphoma research have led to the development of optimized treatment strategies resulting in significantly improved therapeutic outcome.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Weijie L, Andrea G, Malik J. Pathogenesis and pathology of pediatric lymphoma. Brisbane. Exon Publications; 2008. pp.1–26.
[2] Asthana S, Labani S, Mehrana S, Bakhshi S. Incidence of childhood leukemia and lymphoma in India. Pediat Hematol Oncol J. 2018; 3:115–120.
[3] Windebank K. Childhood lymphoma. Indian J Pediatr. 1998; 65:669–680.
[4] Sherief LM, Elsafy UR, Abdelkhalek ER, Kamal NM, Youssef DM, et al. Disease patterns of pediatric non–Hodgkin lymphoma: A study from a developing area in Egypt. Mol Clin Oncol. 2015; 3:139–144.
[5] Zafar S, Sharma RK, Cunningham J, Mahalingam P, Attygalle AD, et al. Current and future best practice in imaging, staging, and response assessment for Non–Hodgkin's lymphomas: the Specialist Integrated Haematological Malignancy Imaging Reporting (SIHMIR) paradigm shift. Clin Radiol. 2021; 76:391.
[6] PDQ Pediatric Treatment Editorial Board. Childhood Hodgkin lymphoma treatment (PDQ®): Health Professional Version. PDQ cancer information summaries. Bethesda (MD): National Cancer Institute (US); 2002.
[7] WHO Classification of Tumours Editorial Board. Haematolymphoid tumours. Lyon: International agency for research on cancer. 5th ed. IARC Publication; 2024.
[8] Sherief LM, Elsafy UR, Abdelkhalek ER, Kamal NM, Elbehedy R, et al. Hodgkin lymphoma in childhood: Clinicopathological features and therapy outcome at 2 Centers From a Developing Country. Medicine. 2015; 94:670.
[9] Mann G, Attarbaschi A, Burkhardt B, Niggli F, Klapper W, et al. Clinical characteristics and treatment outcome of infants with non–Hodgkin lymphoma. Br J Haematol. 2007; 139:443–449.
[10] Gualco G, Klumb CE, Barber GN, Weiss LM, Bacchi CE. Pediatric lymphomas in Brazil. Clinics. 2010; 65:1267–1277.
[11] PDQ Pediatric Treatment Editorial Board. Childhood non Hodgkin lymphoma treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. National Cancer Institute; 2002.
[12] Manipadam MT, Nair S, Viswabandya A, Mathew L, Srivastava A, et al. Non–Hodgkin lymphoma in childhood and adolescence: frequency and distribution of immunomorphological types from a tertiary care center in South India. World J Pediatr. 2011; 7:318–325.
[13] Lee SS, Kim JM, Ko YH, Huh J, Kang CS, et al. Korean Pediatric/Adolescent Lymphoma: Incidence and pathologic characteristics. J Pathol Translat Med. 2010; 44:117–124.
[14] Nakagawa A, Nakamura S, Nakamine H, Yoshino T, Takimoto T, et al. Pathology review for paediatric non–Hodgkin's lymphoma patients in Japan; a report from the Japan association of childhood leukaemia study (JACLS). Eur J Cancer. 2004; 40:725–733.
[15] Huang S, Jin L, Yang J, Duan Y, Zhang M, et al. Characteristics of central nervous system (CNS) involvement in children with non–Hodgkin's lymphoma (NHL) and the diagnostic value of CSF flow cytometry in CNS positive disease. Technol Cancer Res Treat. 2021; 20.
[16] Suh JK, Gao YJ, Tang JY, Jou ST, Lin DT, et al. Clinical characteristics and treatment outcomes of pediatric patients with non–Hodgkin lymphoma in East Asia. Cancer Res Treat. 2020; 52:359–368.
[17] Devine KJ, Trivedi H, Reilly AF. B–lymphoblastic lymphoma in children: A case series from a single institution. J Pediatr Hematol Oncol. 2024; 46:254–258.
[18] Miles RR, Raphael M, McCarthy K, Wotherspoon A, Lones MA, et al. Pediatric diffuse large B–cell lymphoma demonstrates a high proliferation index, frequent c–Myc protein expression, and a high incidence of germinal center subtype: Report of the French–American–British (FAB) international study group. Pediatr Blood Cancer. 2008; 51:369–374.
[19] Dokmanovic L, Krstovski N, Vukanic D, Brasanac D, Rodic P, et al. Pediatric non–Hodgkin lymphoma: A retrospective 14–year experience with Berlin–Frankfurt–Münster (BFM) protocols from a tertiary care hospital in Serbia. Pediatr Hematol Oncol. 2012; 29:109–118.
[20] Prokoph N, Larose H, Lim MS, Burke GAA, Turner SD. Treatment Options for Paediatric Anaplastic Large Cell Lymphoma (ALCL): Current Standard and beyond. Cancers (Basel). 2018; 10:99.
[21] Faizan M, Taj MM, Anwar S, Asghar N, Ahmad A, et al. Comparison of presentation and outcome in 100 pediatric Hodgkin lymphoma patients treated at children hospital, Lahore, Pakistan and Royal Marsden Hospital, UK. J Coll Physicians Surg Pak. 2016; 26:904–907.
[22] Ali N, Mansour M, Khalil E, Ebeid E. Outcome and prognostic factors of pediatric patients with Hodgkin lymphoma: a single–center experience. J Egypt Natl Canc Inst. 2023; 35:29.
[23] Radhakrishnan V, Dhanushkodi M, Ganesan TS, Ganesan P, Sundersingh S, et al. Pediatric Hodgkin Lymphoma Treated at Cancer Institute, Chennai, India: Long–Term Outcome. J Glob Oncol. 2016; 3:545–554.