Orginal Research
2024
June
Volume : 12
Issue : 2
Effect of insulin versus dietary restriction alone on umbilical cord and placental arterio-venous anastomoses in women with gestational diabetes mellitus
Valsalan ES, Jacob J, Kuriachan S, Kumar DM, Rakhesh LR
Pdf Page Numbers :- 174-178
Seema Valsalan E1, Jumi Jacob2, Sanitha Kuriachan2, Mahesh Kumar D3,* and Rakhesh LR4
1Department of Anatomy, PK Das Institute of Medical Sciences, Vaniamkulam, Palakkad, Kerala 679522, India
2Department of Pharmacology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala 680005, India
3Department of Pharmacology, Amrita School of Medicine, Amrita Institute of Medical Sciences, Edappally, Cochin, Kerala 682041, India
4Department of Pharmacology, Dr. Moopen's Medical College, Wayanad, Kerala 673577, India
*Corresponding author: Dr. Mahesh Kumar D, Department of Pharmacology, Amrita School of Medicine, Amrita Institute of Medical Sciences, Edappally, Cochin, Kerala 682041, India. Email: saakethbhagat@gmail.com
Received 10 January 2024; Revised 19 February 2024; Accepted 28 February 2024; Published 6 March 2024
Citation: Valsalan ES, Jacob J, Kuriachan S, Kumar DM, Rakhesh LR. Effect of insulin versus dietary restriction alone on umbilical cord and placental arterio-venous anastomoses in women with gestational diabetes mellitus. J Med Sci Res. 2024; 12(2):174-178. DOI: http://dx.doi.org/10.17727/JMSR.2024/12-33
Copyright: © 2024 Valsalan ES et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Gestational diabetes mellitus (GDM) induces structural alterations in the umbilical cord and placental vessels. This study aims to understand the potential impact of insulin therapy on arteriovenous anastomosis (AVA) in placenta and umbilical cord.
Materials and methods: We collected 52 normal and 59 GDM placentas and umbilical cords from the labour room. GDM specimens were categorized into those treated with diet (GDM-Diet) (n = 23) and those treated with insulin (GDM-Insulin) (n= 36). The umbilical cord was dissected near its insertion to expose the vessels, and arteriovenous anastomoses were documented. Tissues underwent processing and staining for microscopic confirmation of AV anastomosis. Comparison was done between normal and GDM specimens, and notably, between GDM-Diet and GDM-Insulin specimens.
Results: Our examination of 59 GDM and 52 normal placentas with attached umbilical cords revealed AV anastomoses in 24 cases. AV anastomosis was observed in 22 (37.3%, n= 59) GDM cases and 2 (3.8%, n=52) normal cases (p=0.0001). Among GDM-Insulin group umbilical cords, 10 (27.78%, n = 36) exhibited AV anastomoses, while GDM-diet specimens displayed a notably higher proportion, with 12 (52.17%, n = 23) demonstrating AV anastomoses (p = 0.05).
Conclusion: AV anastomoses are markedly more prevalent in the umbilical cords and placentas of individuals with GDM. Interestingly, within the GDM cohort, prevalence is significantly higher in those managed with diet. Our study highlights the association between GDM management strategies and AVC prevalence, emphasizing the importance of considering vascular dynamics in the management of GDM pregnancies.
Keywords: placenta; umbilical cord; fetal- maternal health; gestational diabetes mellitus
Full Text
Introduction
Arteriovenous anastomosis, a direct connection between an artery and a vein bypassing capillaries [1], can occur under both physiological and pathological conditions [2]. Gestational diabetes mellitus (GDM) induces structural changes in all vessels, including those in the umbilical cord and placenta, leading to increased vascular resistance (R) and compromised blood flow [3].
Blood flow through a tissue bed (Q) is determined by the pressure differential (ΔP) across the vascular bed and inversely proportional to the resistance to flow ( [Q = ΔP/R]) [4]. In the context of GDM, fetal vascular insulin resistance has emerged as a significant factor influencing fetal blood flow dynamics by regulating placental vascular tone [5]. This condition adds complexity to the placental vascular system, which is inherently characterized by low resistance [6]. However, GDM introduces pathological changes in placental blood vessels, further exacerbating vascular resistance [6]. A recent comparative study examining GDM placentas against their normal counterparts revealed notable differences in vascular structure, suggesting their potential role in augmenting vascular resistance observed in GDM pregnancies [7].
Introducing a low-resistance arteriovenous fistula (AVF) in parallel to the high-resistance circuit via the tissue capillary bed could facilitate blood bypassing, improving fetal outcomes [8]. Furthermore, an AVF shunt could reduce systemic vascular resistance (SVR) and increase cardiac output (CO) [9]. Hence, arterio-venous anastomosis (AVA) between umbilical or placental vessels could be a mechanism to mitigate vascular resistance, bypass capillary bed flow, and enhance overall blood circulation [10, 11]. Moreover, the size and location of the fistula dictate the flow through the anastomotic circuit, with proximal fistulae having lower resistance compared to distal ones [12].
With this background, we investigated the presence of AV anastomosis in the distal segments of the umbilical cord and placental surface among GDM cases and compared it with normal cases. To understand the potential impact of insulin therapy on placental and umbilical vessel morphology, we specifically explored AVA in GDM mothers treated with diet (GDM-Diet) and those treated with medication (GDM-Insulin).
Materials and methods
The study was conducted at PK Das Institute of Medical Sciences after obtaining Institutional ethics committee approval. During the study period of 4 years from 2017-2021, umbilical cords attached to placentas of 52 normal and 59 GDM mothers, diagnosed according to American Diabetic Association guidelines [13], were collected from the Department of Obstetrics and Gynecology, after obtaining the informed consents from the subjects. Umbilical cords of mothers following normal vaginal deliveries or caesarean section, within 36th to 40th week of gestation, were included in the study and those having any other complications other than GDM as well as normal mothers with family history of diabetes were excluded from the participant group. GDM specimens were again grouped into GDM treated with diet (GDM-Diet) and GDM treated with Insulin (GDM-Drug). Specimens were thoroughly washed to clear blood and mucus and were preserved in 10% formalin for fixation [14]. After 2-3 days the cord was dissected near its insertion to expose the vessels and was traced on to the placenta to look for any arteriovenous anastomosis [15]. AV anastomosis tissues were then processed and stained with H& E staining according to standard institutional protocols [14, 15]. The stained slides were examined to confirm the AV anastomosis and images were captured under Olympus Cx2li microscope. The proportion of AV anastomosis was calculated among the groups and Chi-square analysis was performed using SPSS version 23.
Statistical analysis
The sample size for this study was calculated using the formula (Z α/2 + Z β)² x 2 SD² / d², where Z α/2 represents the α error (1.96), Z β represents the β error (0.84), SD is the average of the standard deviations (sd1 + sd2 / 2), and d is the difference between the means (Mean1 - Mean2), as described by Alam et al. (2014) [15]. This resulted in a required sample size of 50 umbilical cords per group. Proportional analyses were performed between normal and GDM specimens, and particularly, between GDM-Diet and GDM-Insulin specimens using Chi-square analysis via SPSS version 23.
Results
Examination of 59 umbilical cords from mothers with gestational diabetes mellitus (GDM) and 52 from mothers without GDM, found out that arteriovenous (AV) anastomoses were present in 24 cases. Among the GDM specimens, 22 (37.3%) cords showed AV anastomosis, whereas only 2 (3.8%) cords from the normal group had AV anastomosis (Table 1).
Table 1: Comparison of arteriovenous anastomosis among GDM and normal placenta.
|
Arteriovenous communication
|
Group
|
Total
|
|
GDM n(%)
|
Normal n(%)
|
|
Absent
|
37(62.7)
|
50(96.2)
|
87
|
|
Present
|
22(37.3)
|
2(3.8)
|
24
|
|
Total
|
59(100.0)
|
52(100.0)
|
111
|
Chi square test p value<0.001
On comparing, 27.78% (10, n=36) of umbilical cords among GDM-Insulin, and 52.17% (12, n=23) cords in GDM-Diet group displayed AV anastomosis, an observation deemed statistically significant (P value< 0.05) (Table 2).
Table 2: Comparison of arteriovenous anastomosis among placentas of GDM mothers managed by insulin and diet.
|
Arteriovenous communication
|
Group
|
Total
|
|
GDM - Diet (%)
|
GDM- Insulin (%)
|
|
Present
|
12 (52.17%)
|
10 (27.78%)
|
33
|
|
Absent
|
11 (47.83%)
|
26 (72.22%)
|
36
|
|
Total
|
23 (100%)
|
36(100%)
|
59
|
Chi square test p value= 0.05
These AV anastomoses were predominantly located near the cord insertion site and were mostly of the fenestrated type, as depicted in Figure 1. Microscopic examination confirmed the presence of AV anastomosis tissue, as illustrated in Figure 2.
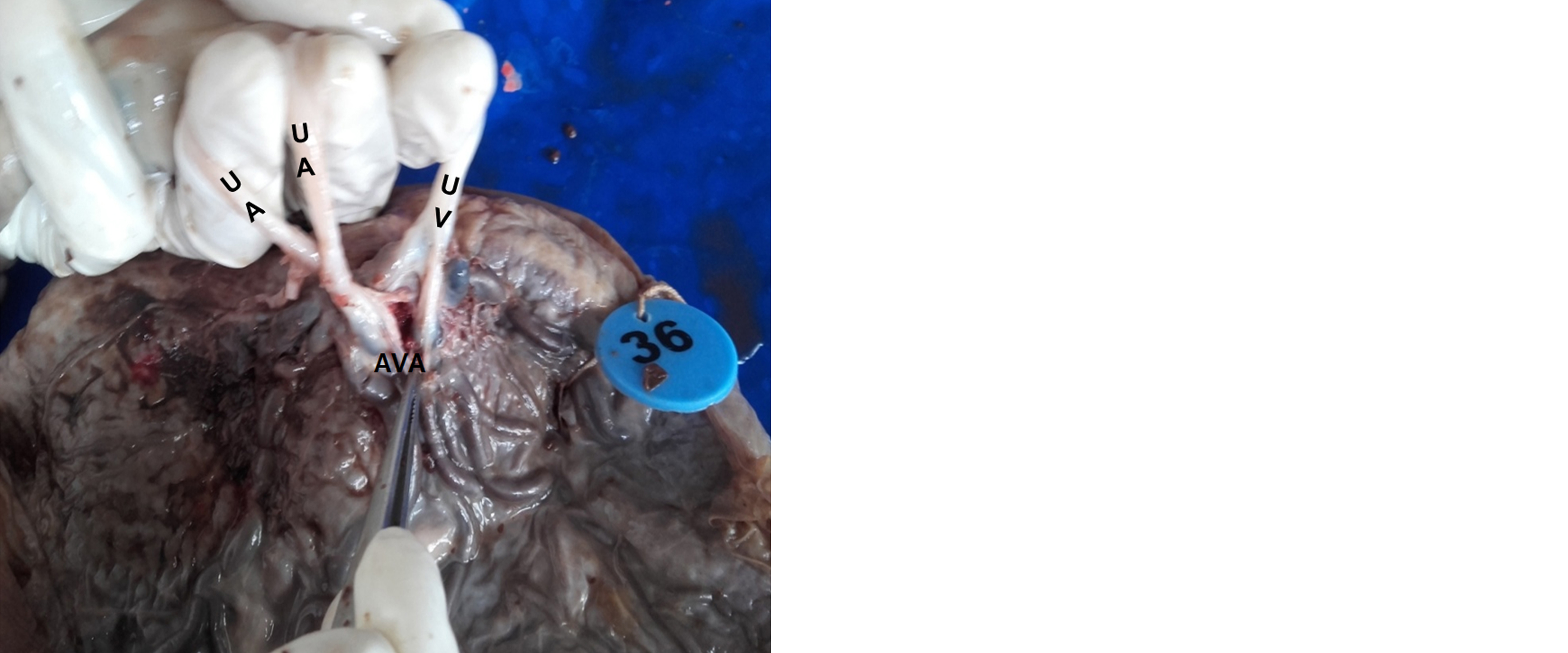
Figure 1: Gross: Arteriovenous anastomosis among umbilical artery and vein.
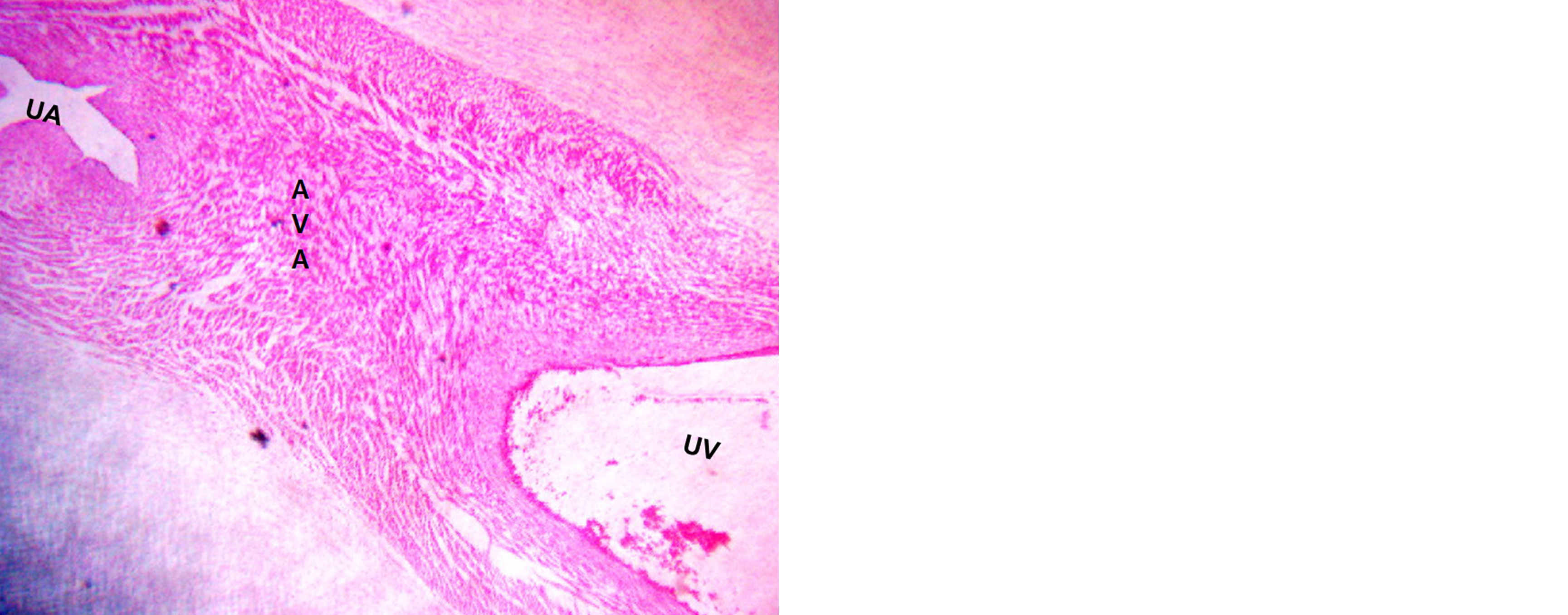
Figure 2: Histology: Arteriovenous anastomosis among umbilical artery and vein.
Discussion
Arteriovenous malformation (AVM) is a rare congenital vascular anomaly characterized by direct blood flow from an artery to a vein bypassing the capillary network. The existing body of literature exhibits a conspicuous dearth concerning an exhaustive exploration of arteriovenous (AV) anastomosis. Notably, this phenomenon has been extensively scrutinized exclusively in the context of multiple pregnancies, employing diverse methodologies to anticipate potential complications.
Umbilical AVMs are exceptionally rare, with only a few documented cases in neonates, typically reported through case studies [16,17]. In most instances, umbilical AVMs are discovered incidentally during assessments for conditions like umbilical hernia or abdominal murmurs. However, they can also manifest suddenly with symptoms such as hemorrhage or high-output cardiac failure [18].
To date, the presence of AV anastomosis in placentas affected by Gestational Diabetes Mellitus (GDM) remains unexplored. The current study unveils a statistically significant prevalence of AV anastomosis in GDM-affected placentas when juxtaposed against their normal counterparts, despite the maintenance of maternal blood sugar levels within the normal range through Insulin/Diet management.
Within GDM cases, a noteworthy disparity in the occurrence of AV anastomosis is discerned between placentas managed with GDM-Diet and those managed with GDM-Insulin, decisively favoring the primacy of Insulin treatment over diet management for GDM mothers. The intricacies of a two-way blood shunt are distinctly viable within superficial AV anastomotic connections on the chorionic plate [19]. Detecting AV anastomosis during the antenatal phase proves feasible with anteriorly positioned placentas but presents challenges in posteriorly situated placentas [20].
Notably, arterial blood flow through an AV anastomosis may transiently diminish effective arterial volume, precipitating impaired venous return distal to the anastomosis, leading to venous congestion and edema [21]. In the present study, GDM placentas exhibited a conspicuous presence of AV anastomosis on the chorionic surface.
Arterial compliance (C), which denotes the vessel's ability to accommodate volume changes in response to pressure alterations, plays a crucial role in vascular physiology. AV anastomosis, by facilitating the direct flow between arteries and veins, serves to effectively reduce pressure, lower resistance, and enhance compliance, as noted by Palatini et al [22], This effect is supported by findings from animal models, such as the canine model, where the creation of arteriovenous fistulas (AVFs) has demonstrated similar outcomes [23].
The presence of AV anastomosis may have additional benefits in conditions like gestational diabetes mellitus (GDM), where the umbilical artery's vessel wall is known to exhibit stiffness [24]. In such cases, AV anastomosis could potentially act as a protective mechanism, mitigating high-pressure flow and improving arterial compliance. Moreover, by enhancing contractility and reducing vascular resistance, AV anastomosis may further contribute to improved vascular emptying [25]. Thus, from this perspective, the presence of AV anastomosis could be seen as a compensatory mechanism to counteract the adverse effects of vascular stiffness associated with conditions like GDM.
From an alternative perspective, proximal arteriovenous shunting, a characteristic of AV anastomosis, redirects blood flow away from the high-pressure capillary bed, leading to decreased distal blood flow. This phenomenon can be particularly problematic in individuals with conditions like peripheral vascular disease or diabetes mellitus [26, 27]. In the context of GDM, placental ischemic-hypoxia manifestations may arise from low-pressure proximal AV anastomosis on the placental surface, ultimately resulting in deep placental tissue hypoxic infarction [28].
Offspring born to mothers with GDM often experience heightened hypoxia and show elevated levels of glucose and hemoglobin, despite their mothers maintaining normoglycemic profiles [29, 30]. The observed differences in oxygen content and partial pressure of oxygen (PO2) between the umbilical vein and artery suggest impaired placental exchange, which may be exacerbated by AV anastomosis-induced placental ischemia. Thus, in the context of GDM, AV anastomosis could contribute to compromised placental function and adverse fetal outcomes, highlighting the intricate interplay between vascular anomalies and maternal-fetal health.
The complex interactions between various types of anastomoses and their effects on shared placental territories and arteriovenous anastomotic patterns play a significant role in influencing fetal growth [31]. This insight emphasizes the importance of understanding the intricate vascular dynamics within the placenta and their implications for fetal development.
The contrasting prevalence of AVC between GDM management groups suggests that the method of glycemic control may influence vascular adaptations in GDM. It is plausible that dietary interventions, while effective in controlling blood glucose levels, may also influence vascular physiology, potentially leading to the development of AVC. Understanding the relationship between GDM management strategies and AVC prevalence is crucial for optimizing maternal-fetal outcomes in GDM pregnancies. Longitudinal studies tracking maternal and fetal outcomes in relation to AVC prevalence could provide valuable insights into the clinical significance of AVC in GDM pregnancies.
Future scope
Future research in the field of placental vascular dynamics and its implications for maternal-fetal health, particularly in the context of conditions like GDM, holds significant promise for advancing our understanding and improving clinical management strategies. To further enhance the depth and scope of future investigations, expanding the study cohort to include a larger sample of GDM patients would be advantageous. Additionally, integrating advanced techniques such as Doppler ultrasound, angiographic methods, or placental injection techniques could provide more comprehensive insights into placental vascular dynamics and their association with GDM.
Limitations: Due to the limited sample size in our study, the findings may not possess the robustness necessary for definitive conclusions. To address this limitation, future research endeavors should prioritize multi-center studies involving larger patient cohorts. Such initiatives would facilitate a more thorough and precise evaluation of disease characteristics and outcomes, thereby improving the reliability and applicability of the findings.
Conclusion
In our study, we found that a higher proportion of GDM participants managed through diet exhibited AVC compared to those managed with insulin. This finding raises several important considerations and prompts further discussion. Further research is warranted to better understand the clinical significance of AVC and its implications for maternal-fetal health in the context of GDM.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temp (Austin, Tex). 2016; 3:92–103.
[2] Al-Ofi E, Alrafiah A, Maidi S, Almaghrabi S, Hakami N. Altered expression of angiogenic biomarkers in pregnancy associated with gestational diabetes. Int J Gen Med. 2021; 14:3367–75.
[3] Cunningham FG, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, et al. Diabetes mellitus. In: Williams Obstetrics, 26e. New York, NY: McGraw Hill; 2022.
[4] Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol. 2017; 7:321–382.
[5] Cornejo M, Fuentes G, Valero P, Vega S, Grismaldo A, et al. Gestational diabesity and foetoplacental vascular dysfunction. Acta Physiol. 2021; 232:e13671.
[6] Li H-P, Chen X, Li M-Q. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013; 6:650–659.
[7] Liang X, Zhang J, Wang Y, Wu Y, Liu H, et al. Comparative study of microvascular structural changes in the gestational diabetic placenta. Diabetes Vasc Dis Res. 2023; 20:14791641231173628.
[8] Holman E. Abnormal arteriovenous communications. Great variability of effects with particular reference to delayed development of cardiac failure. Circulation. 1965; 32:1001–1009.
[9] Clemmer JS, Pruett WA, Hester RL, Lohmeier TE. Preeminent role of the cardiorenal axis in the antihypertensive response to an arteriovenous fistula: an in silico analysis. Am J Physiol Heart Circ Physiol. 2019; 317:H1002–H1012.
[10] Umur A, van Gemert MJC, Nikkels PGJ, Ross MG. Monochorionic twins and twin–twin transfusion syndrome: The protective role of arterio-arterial anastomoses. Placenta. 2002; 23:201–209.
[11] Haimovici H, Steinman C, Caplan LH. Role of arteriovenous anastomoses in vascular diseases of the lower extremity. Ann Surg. 1966; 164:990–1002.
[12] Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2011; 26:3296–302.
[13] American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003; 26:103–105.
[14] Kim S, Christopher L, Bancroft JD . Bancroft’s theory and practice of histological techniques. 8t edn. Elsevier, 2018.
[15] Alam M, Momen M, Sultana A, Hassan SM. Gross and histomorphologic study of the umbilical cord in pre-gestational diabetes mellitus and gestational diabetes mellitus. Bangladesh J Anat. 2015; 12:25–29.
[16] Ester GH, Aguado-Del HA, Gema MM, Carlos DAJ; Manuel RSL. A rare complex case of congenital umbilical arteriovenous malformation and review of literature. AJP Rep. 2016; 06:e216–e221.
[17] Suzui I, Masuyama H, Hirano Y, Nishida T, Hayata K, et al. Prenatal diagnosis of umbilical arteriovenous malformation. J Matern neonatal Med. 2017; 30:85–87.
[18] Sherer DM, Al-Haddad S, Cheng R, Dalloul M. Current perspectives of prenatal sonography of umbilical cord morphology. Int J Womens Health. 2021; 13:939–971.
[19] Lopriore E, Slaghekke F, Middeldorp JM, Klumper FJ, van Lith JM, et al. Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. J Vis Exp. 2011; 55:e3208.
[20] Sau A, Weber M, Shennan AH, Maxwell D. Antenatal detection of arteriovenous anastomoses in monochorionic twin pregnancy. Int J Gynaecol Obstet Off organ Int Fed Gynaecol Obstet. 2008; 100:56–59.
[21] Bertog SC, Kolmer C, Kleschnew S, Franke J, Wunderlich N, et al. Percutaneous femoral arteriovenous shunt creation for advanced chronic obstructive pulmonary disease: a single-center safety and efficacy study. Circ Cardiovasc Interv. 2012; 5:118–126.
[22] Palatini P, Casiglia E, Gąsowski J, Głuszek J, Jankowski P, et al. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag. 2011; 7:725–739.
[23] Guyton AC, Sagawa K. Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. Am J Physiol. 1961; 200:1157–1163.
[24] Goshu BT. Histopathologic impacts of diabetes mellitus on umbilical cord during pregnancy. Pediatr Heal Med Ther. 2022; 13:37–41.
[25] MacRae JM, Pandeya S, Humen DP, Krivitski N, Lindsay RM. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis [Internet]. 2004;43(5):e21.1-e21.6.
[26] Dikow R, Schwenger V, Zeier M, Ritz E. Do AV fistulas contribute to cardiac mortality in hemodialysis patients? Semin Dial. 2002; 15:14–7.
[27] Miles AM. Vascular steal syndrome and ischaemic monomelic neuropathy: two variants of upper limb ischaemia after haemodialysis vascular access surgery. Nephrol Dial Transplant. 1999; 14:297–300.
[28] Lei J, Zhao M, Li L, Ji B, Xu T, et al. Research progress of placental vascular pathophysiological changes in pregnancy-induced hypertension and gestational diabetes mellitus. Front Physiol. 2022;13:954636.
[29] Taricco E, Radaelli T, Rossi G, Santis MSN, Bulfamante GP, et al. Effects of gestational diabetes on fetal oxygen and glucose levels in vivo. BJOG. 2009; 116:1729–35.
[30] Hufnagel A, Grant ID, Aiken CEM. Glucose and oxygen in the early intrauterine environment and their role in developmental abnormalities. Semin Cell Dev Biol. 2022; 131:25–34.
[31] Lewi L, Cannie M, Blickstein I, Jani J, Huber A, et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am J Obstet Gynecol. 2007; 197:587.e1–8.