Full Text
Introduction
Intracranial wide neck aneurysms are an important subset of intracranial aneurysms which are defined by neck diameters > 4mm or dome: neck ratio <2 [1]. Simple endovascular coiling alone may not be sufficient due to their wide neck [2]. Extra-vascular managements of these aneurysms also pose increased risks of complications. With the advent of newer endovascular techniques e.g., stent assisted coiling, balloon assisted coiling, dual micro-catheter coiling method, flow diverter, aneurysmal neck reconstruction devices etc., there has been a revolution in endovascular management of wide neck aneurysms [3].
Digital subtraction angiography with 3D Digital Rotational Angiography has good spatiotemporal resolution for understanding of the pathology and confirmation of diagnosis and also provides unique possibilities to perform direct endovascular embolization in the same setting [4]. In this retrospective study, we have assessed the demographic profile, clinical presentation and endovascular management of 12 such patients presenting to our department in a tertiary care centre of Eastern India over a 1-year period.
Materials and methods
A retrospective descriptive study was conducted at the Department of Radiodiagnosis, IPGME & R and SSKM Hospital from June 2021 to May 2022 after getting approval from institutional ethics committee. 12 consecutive patients of intracranial wide neck aneurysms (Suspected clinically or suggested by Computed Tomographic Angiography/ Magnetic Resonance Angiography) who attended Neuromedicine & Neurosurgery Outpatient Department and were referred to our department for a period of 12 months and confirmed by Diagnostic Digital Subtraction Angiography were included in the study. Therapeutic Endovascular Embolization was done on these cases. The angiographic results were classified according to the Raymond-Roy classification system as follows: Class 1, no filling of the aneurysm neck or dome; Class 2, residual filling of the neck, but not the dome; and Class 3, residual filling of the neck and dome.
Inclusion criteria: Patients with intracranial aneurysms were included in the study if the aneurysm neck diameter was greater than 4 mm or if the dome-to-neck ratio was less than 2, regardless of whether the aneurysms were ruptured or unruptured.
Exclusion criteria: Aneurysms that did not meet the criteria for wide-neck aneurysms, such as saccular, fusiform, blister, or pseudoaneurysms, were excluded. Additionally, wide-neck aneurysms associated with other intracranial vascular pathologies, such as arteriovenous malformations, were also excluded from the study.
Results
Among the 12 patients treated, 10 were female and 2 were male patients. In 5 cases, stent assisted coiling was used alone. We used closed cell enterprise stents for this purpose and both jailing and crossing techniques were used. In 3 cases dual micro-catheter techniques were used. We used prowler select plus micro-catheters for this purpose. Two cases were managed by balloon assisted coiling by hyperglide balloon occlusion system. In case of ruptured internal carotid artery (ICA) paraophthalmic aneurysm forming a pseudoaneurysm, partial coiling was done in conjunction with of dual micro-catheter method. In another case ICA para-Pcom aneurysm, balloon assisted coiling was used in conjunction with dual micro-catheter technique. The cases of this series are summarized in Table 1.
Table 1: Demographic profile, clinical presentation, and endovascular management of 12 patients with wide-neck intracranial aneurysms admitted to the Department of Radiodiagnosis.
|
Age (Years)
|
Sex
|
Clinical Presentation
|
Imaging Findings
|
Management
|
|
55
|
F
|
Sudden onset severe headache and loss of consciousness
|
Left paraophthalmic tandem ICA aneurysm, directed postero-inferiorly, measuring [Neck (N), Dome (D), Height (H) respectively] 1.6mm x 2.93mm x 2.99 mm and 1.43mm x 1.49mm x 1.7 mm. Modified Fischer Grade 3 SAH in NCCT
|
Managed by Hyperglide occlusion balloon assisted coiling (Axium prime coils) embolization. Raymond Roy Class II Occlusion was achieved.
|
|
54
|
M
|
Loss of consciousness
|
Bilobed aneurysm in the basilar artery [4.2mmx5.8mmx7mm - N, D, H] and Left P1 PCA [2.1mmx3.8mmx3mm - N, D, H], both directed superomedially, the larger aneurysm had a teat on its dome, indicating its ruptured status. Modified Fischer scale 4 SAH was present.
|
Enterprise stent assisted coil embolization was done by jailing technique with orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved.
|
|
56
|
F
|
Headache, Visual problems, Pupillary abnormality (left sided ptosis and unequal pupils, the left pupil was more constricted than the right)
|
No SAH in NCCT. DSA revealed Left C4 ICA Para Posterior Communicating Artery Aneurysm, directed posterolateroinferiorly, measuring 4.1mm x7mmx 5.6mm (N, D, H). The aneurysm had a teat on its dome, indicating impending rupture.
|
Coiling by dual microcatheter technique with two Prowler select plus microcatheters orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved
|
|
66
|
F
|
Loss of consciousness, Pupillary abnormality
|
Right Para Pcom C4 ICA Aneurysm,measuring 4.3 mm x 6.8 mm x 9.03 mm (N, D, H) and directed posteroinferiorly and laterally. Modified Fischer Scale Grade 3 SAH
|
Coiling by dual microcatheter technique with two Prowler select plus microcatheters orbit galaxy Frame and Fill coils. Raymond Roy Class III A Occlusion was achieved
|
|
61
|
M
|
Sudden onset severe headache, loss of consciousness and loss of vision in left eye (PL +)
|
Modified Fischer scale 3 SAH. DSA revealed anterolaterally directed large paraophthalmic aneurysm in the Left C6 ICA [5.8 mm x15.3 mm x15.1 mm - Neck, Dome and Height, respectively].
|
Partial coiling of the pseudoaneurysm and coiling of the aneurysm by dual microcatheter technique with two Prowler select plus microcatheters orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved.
|
|
43
|
F
|
Sudden onset severe headache, loss of consciousness and vertigo
|
No SAH in NCCT. Left V4 vertebral artery aneurysm, directed posterolaterally and superiorly, measuring 4.25 mm x 5.22 mm x 3.57 mm (N, D, H).
|
Enterprise stent assisted coil embolization was done by jailing technique with orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved.
|
|
54
|
F
|
Sudden onset severe headache and loss of consciousness
|
NCCT Brain revealed no SAH. Posterolaterally and inferiorly directed Right C7 ICA Aneurysm measuring 2.8mm x 4.1 mm x 4.29 mm (N, D, H).
|
Coiling by dual microcatheter technique with two Prowler select plus microcatheters orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved
|
|
51
|
F
|
Sudden onset severe headache, loss of consciousness and right sided weakness
|
NCCT revealed Modified Fischer scale 3 SAH. DSA revealed Anterolaterally directed Left C4 ICA Aneurysm measuring 22 mm x 4.3 mm x 4.1 mm (N, D, H).
|
Enterprise stent assisted coil embolization was done by crossing technique with orbit galaxy Frame and Fill coils. Raymond Roy Class III A Occlusion was achieved.
|
|
40
|
F
|
Sudden onset severe headache and loss of consciousness
|
Modified Fischer scale 2 SAH in NCCT. DSA revealed Antero- superiorly directed Anterior communicating artery aneurysm measuring 3.8 mm x 2.6 mm x 4.8 mm (N, D, H)
|
Enterprise stent assisted coil embolization was done by jailing technique with orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved.
|
|
55
|
F
|
Occasional Headache, diminished vision in Right eye and papillary abnormality
|
No SAH in NCCT. Posteriorly directed Right Para Pcom C7 ICA aneurysm, measuring 3.2 mm x 3.8 mm x 5.5 mm (N, D, H).
|
Coiling by dual microcatheter technique with two Prowler select plus microcatheters and Hyperglide occlusion balloon assisted coiling done with orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved
|
|
52
|
F
|
Sudden onset severe headache and loss of consciousness
|
Modified Fischer scale 4 SAH was present in NCCT. Wide neck basilar top aneurysm directed superiorly, measuring 4.8 mm x1.4 mm x 1.9 mm (N, D, H).
|
Enterprise stent assisted coil embolization was done by jailing technique with orbit galaxy Frame and Fill coils. Raymond Roy Class II Occlusion was achieved.
|
|
39
|
F
|
Sudden onset severe headache and loss of consciousness
|
Modified Fischer Grade 3 SAH in NCCT. Right paraophthalmic ICA aneurysm directed posterosuperiorly, measuring 3.6mm x 3.93mm x 5.98 mm. (N, D, H).
|
Hyperglide occlusion balloon assisted coiling (Axium prime coils) embolization. Raymond Roy Class III A Occlusion was achieved.
|
Illustrative cases
Case 1: A 55-year-old female patient with a history of diabetes and hypertension presented to the emergency department with complaints of sudden-onset headache, loss of consciousness, and disorientation. On examination, her Glasgow Coma Scale (GCS) score was E2V1M5, SpO₂ was 97%, and she exhibited tonic-clonic movements in the limbs. The patient was stabilized, and a combination of Duolin and Budecort (2:1) was administered. A non-contrast CT (NCCT) of the brain was advised. The NCCT of the brain revealed a thick (>1 mm) subarachnoid hemorrhage (SAH) in the left frontal region, without evidence of intraventricular hemorrhage, corresponding to Grade 3 on the Modified Fisher Scale. She was admitted and started on mannitol and levetiracetam. A CT angiography was subsequently performed, which revealed a left paraophthalmic aneurysm. She was then referred to the Interventional Neuroradiology department for diagnostic neuroangiography and possible endovascular management.
Preoperative evaluation before the diagnostic digital subtraction angiography (DSA) revealed a GCS of E4V5M6, good peripheral pulses, and occasional tachycardia. The DSA identified two tandem paraophthalmic aneurysms arising from the left internal carotid artery (ICA), directed posteriorly and posteroinferiorly. The first aneurysm measured 1.6 mm × 2.93 mm × 2.99 mm (neck, dome, and height, respectively), and the second measured 1.43 mm × 1.49 mm × 1.7 mm. The second aneurysm had a teat-like projection on its dome, suggestive of a previous rupture (Figure 1a). The case was managed with coil embolization using Hyperglide balloon assistance and Axium Prime coils (Figure 1b and 1c). Raymond-Roy Occlusion Classification Class II occlusion was achieved. Postoperatively, the patient remained stable and was transferred to the Neuro ICU for observation over the next 24 hours. She was discharged on the 4th postoperative day.
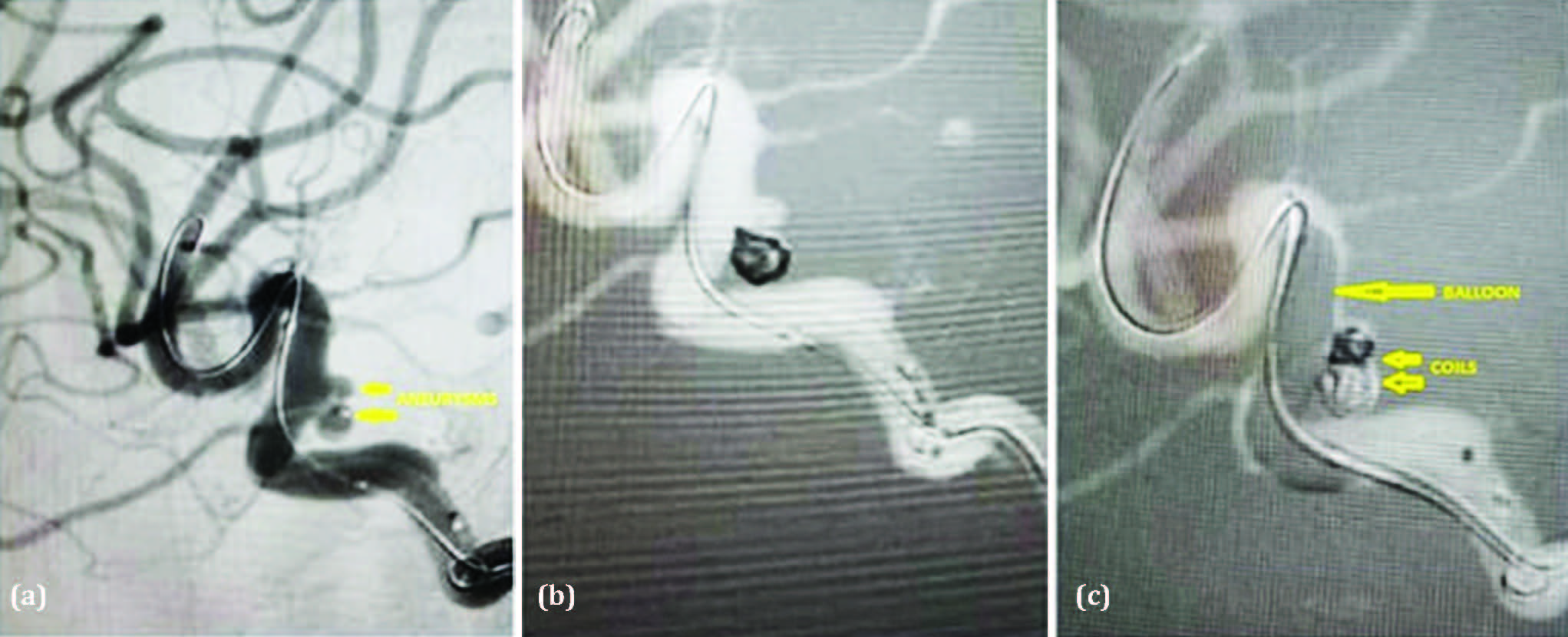
Figure 1: (a) Digital subtraction angiogram lateral view of left ICA showing two tandem paraophthalmic aneurysms arising from the left ICA, directed posteriorly and posteroinferiorly. The first aneurysm measured 1.6mm x 2.93mm x 2.99mm (Neck, Dome, and Height, respectively) and 1.43mm x 1.49mm x 1.7mm (Neck, Dome, and Height, respectively). The second aneurysm showed a teat on its dome, indicating a previous rupture. (b & c) Balloon-assisted coil embolization using Hyperglide balloon assistance and Axium Prime coils.
Case 2: A 54-year-old male patient, non-diabetic and hypertensive, presented to the Medicine OPD with a history of a fall in the bathroom followed by sudden-onset loss of consciousness. On examination, his Glasgow Coma Scale (GCS) score was E2V2M6, and his SpO₂ was 99%. The patient was stabilized, administered Duolin and Budecort (2:1), and a non-contrast CT (NCCT) of the brain was recommended. The NCCT brain revealed a thick (>1 mm) subarachnoid hemorrhage (SAH), classified as Grade 4 on the Modified Fisher Scale, along with intraventricular hemorrhage and subdural hemorrhage in the occipital region. He was admitted and started on mannitol and levetiracetam. A time-of-flight (TOF) MR angiography was performed, which indicated two aneurysms in the basilar artery and P1 segment of left posterior cerebral artery (Figure 2a). He was subsequently referred to the Interventional Neuroradiology department for diagnostic neuroangiography and further endovascular management.
Digital Subtraction Angiography (DSA) revealed a bilobed aneurysm in the basilar artery, measuring 4.2 mm × 5.8 mm × 7 mm (neck, dome, and height, respectively), and another aneurysm in the P1 segment of the left posterior cerebral artery (PCA), measuring 2.1 mm × 3.8 mm × 3 mm (Figure 2b). Both aneurysms were directed superomedially. The larger basilar aneurysm had a teat-like projection on its dome, suggestive of a ruptured state. Endovascular treatment was performed using Enterprise stent-assisted coiling with the jailing technique, employing Orbit Galaxy Frame and Fill coils (Figure 2c and 2d). Raymond-Roy Occlusion Classification Class II occlusion was achieved. Postoperatively, the patient remained stable and was transferred to the Neuro ICU for monitoring over the next 24 hours. He was discharged on the 7th postoperative day. The relatively delayed discharge was due to the development of hospital-acquired pneumonitis.
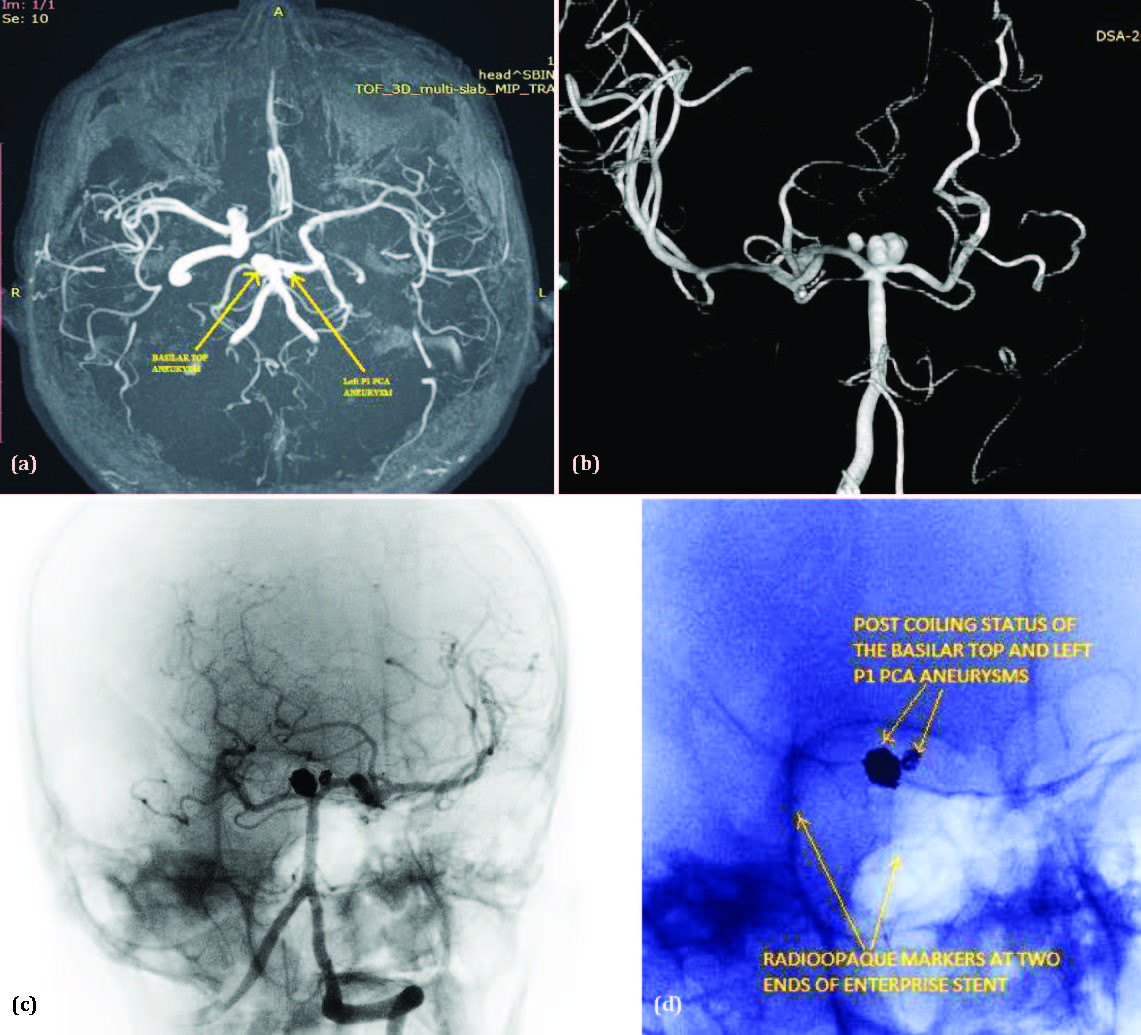
Figure 2: (a) Time of flight MR angiogram of brain revealed aneurysm of basilar artery and left P1 posterior cerebral artery aneurysm. (b) 3D Rotational digital subtraction angiogram of brain revealed a bilobed aneurysm in the basilar artery [4.2mm x 5.8mm x 7mm - Neck, Dome, and Height, respectively] and the left P1 PCA [2.1mm x 3.8mm x 3mm - Neck, Dome, and Height, respectively], both directed superomedially. The larger aneurysm exhibited a teat on its dome, indicating a previous rupture. (c & d) Post-coiling digital subtraction angiogram superimposed on native image (2c), and zoomed view of native image (2d) showing Enterprise stent-assisted coiling with the jailing technique, employing Orbit Galaxy Frame and Fill coils.
Case 3: A 55-year-old female patient, non-diabetic and non-hypertensive, presented to the Neuromedicine OPD with complaints of transient visual disturbances, drooping of the left eyelid, and intermittent headaches. On examination, her Glasgow Coma Scale (GCS) score was E4V5M6, and her SpO₂ was 99%. Clinical examination revealed left-sided ptosis and anisocoria, with the left pupil being more constricted than the right. A non-contrast CT (NCCT) brain was advised. The NCCT brain did not reveal any evidence of subarachnoid hemorrhage (SAH). A CT angiography was subsequently performed, which revealed an aneurysm in the left internal carotid artery (ICA). She was then referred to the Interventional Neuroradiology department for diagnostic neuroangiography and further endovascular management.
Preoperative assessment before the diagnostic digital subtraction angiography (DSA) revealed a GCS score of E4V5M6, good peripheral pulses, and occasional tachycardia. The DSA showed a para-communicating segment aneurysm arising from the C4 segment of the left ICA, directed postero-latero-inferiorly, measuring 4.1 mm × 7 mm × 5.6 mm (neck, dome, and height, respectively). A teat-like projection on the dome of the aneurysm suggested an impending rupture (Figure 3a). The aneurysm was treated by coil embolization using the dual microcatheter technique with two Prowler Select Plus microcatheters and Orbit Galaxy Frame and Fill coils (Figure 3b, 3c and 3d). Raymond-Roy Occlusion Classification Class II occlusion was achieved. Postoperatively, the patient remained stable and was transferred to the Neuro ICU for 24-hour monitoring. Partial improvement in ptosis was noted, along with a slight increase in pupillary diameter. She was discharged on the 5th postoperative day.
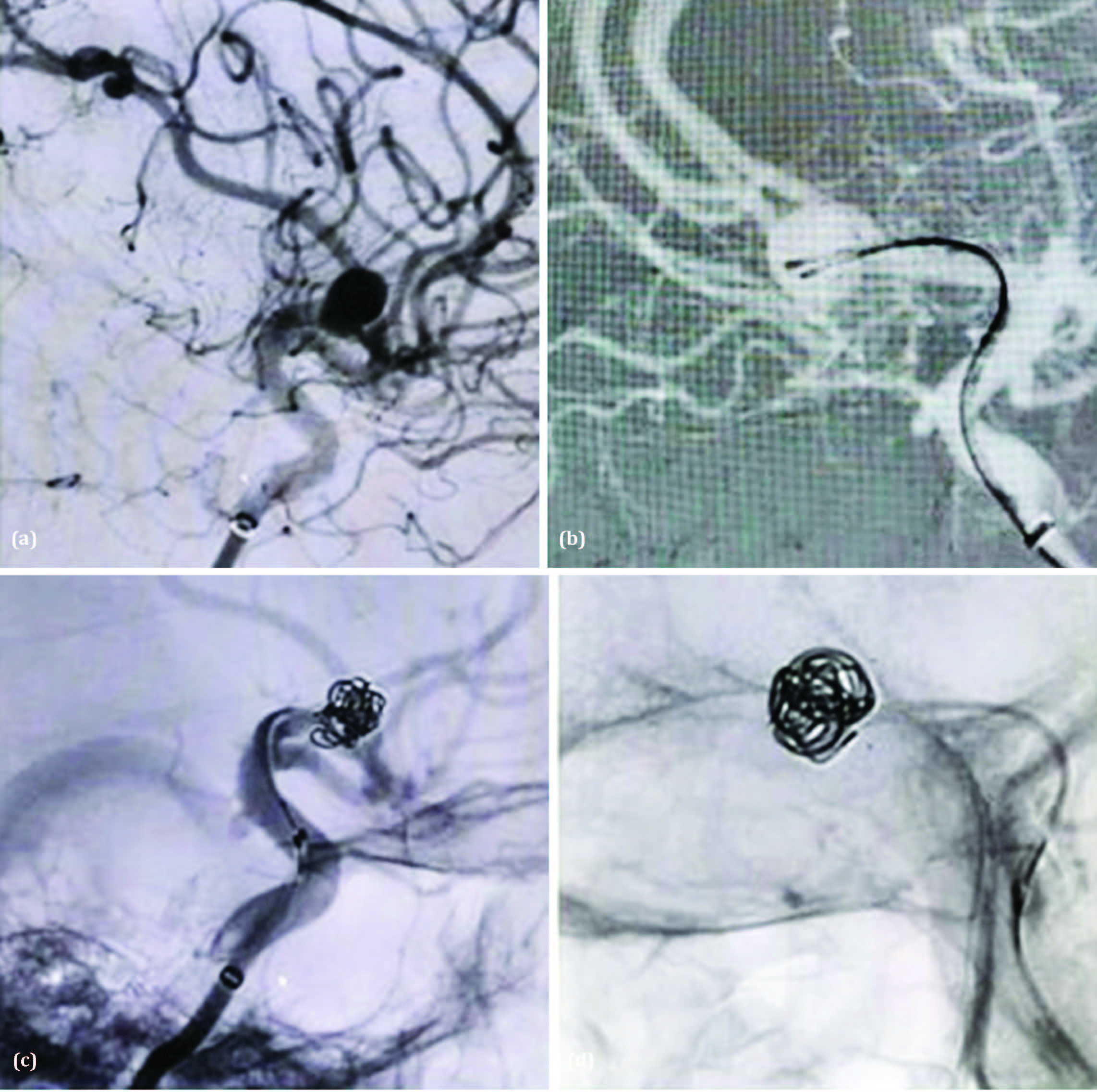
Figure 3: (a) Digital subtraction angiogram lateral view of left ICA showing a para-communicating segment aneurysm arising from the C4 segment of the left ICA, directed postero-latero-inferiorly, measuring 4.1 mm × 7 mm × 5.6 mm (neck, dome, and height, respectively). A teat-like projection on the dome of the aneurysm suggested an impending rupture. (b-d) Coiling using the Dual Microcatheter Technique with two Prowler Select Plus catheters and Orbit Galaxy Frame and Fill coils.
Case 4: A 61-year-old male patient, non-diabetic and hypertensive, presented to the emergency department with complaints of sudden-onset severe headache, loss of consciousness, and loss of vision in the left eye. On examination, his Glasgow Coma Scale (GCS) score was E2V2M5, and his SpO₂ was 96%. The patient was stabilized and administered Duolin and Budecort (2:1). A non-contrast CT (NCCT) brain was advised. The NCCT brain revealed a thick (>1 mm) subarachnoid hemorrhage in the basal cisterns and left Sylvian fissure, classified as Grade 3 on the Modified Fisher Scale, without evidence of intraventricular hemorrhage. Ocular examination showed perception of light (PL+) in the left eye. He was admitted and started on mannitol and levetiracetam. A CT angiography was performed, which revealed a large pseudoaneurysm around and arising from the left internal carotid artery (ICA). The patient was then referred to the Interventional Neuroradiology department for diagnostic neuroangiography and further endovascular management.
Digital Subtraction Angiography (DSA) revealed a large anterolaterally directed paraophthalmic aneurysm in the C6 segment of the left ICA, measuring 5.8 mm × 15.3 mm × 15.1 mm (neck, dome, and height, respectively). The case was managed with partial coiling of the pseudoaneurysm and coiling of the aneurysm using the dual microcatheter technique with two Prowler Select Plus microcatheters and Orbit Galaxy Frame and Fill coils (Figure 4a, 4b and 4c). Raymond-Roy Occlusion Classification Class II occlusion was achieved. Postoperatively, the patient remained stable and was monitored in the Neuro ICU for the next 24 hours. He was discharged on the 6th postoperative day from the Interventional Neuroradiology department and referred to the Ophthalmology department for further evaluation and management of his visual impairment. However, there was no significant improvement in vision at the time of discharge.
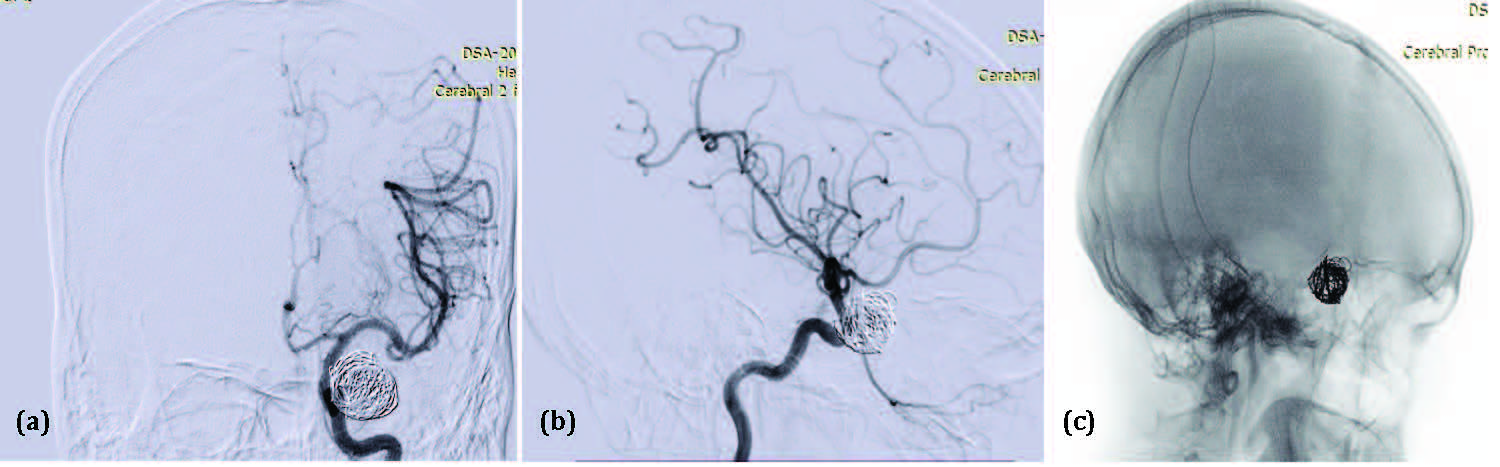
Figure 4: Digital subtraction angiogram Antero-posterior view (a), Lateral view (b) of left ICA and native image in oblique view (c) shows partial coiling of the pseudoaneurysm in the subarachnoid space, followed by coiling of the left paraophthalmic ICA aneurysm using the dual microcatheter technique with two Prowler Select Plus microcatheters and Orbit Galaxy Frame and Fill coils.
Discussion
Cloft et al. observed that endovascular therapy for aneurysms with a dome-to-neck ratio greater than 1.5 became feasible with the advent of advanced, complex coil designs [5]. Wide-neck bifurcation aneurysms remain challenging to treat, whether through endovascular or surgical approaches [6]. Endovascular techniques are increasingly preferred over microsurgical clipping for treating cerebral aneurysms, as they are associated with reduced morbidity and mortality in appropriately selected patients [7]. To determine the most suitable treatment approach, a multidisciplinary evaluation of each aneurysm is essential [8].
The classical endovascular coiling approach presents challenges in treating aneurysms with complex morphologies—such as fusiform, large or giant, and wide-neck aneurysms, or those with an unfavorable relationship between the neck, dome, and parent artery [9]. In cases of ruptured wide-neck aneurysms, balloon-assisted coiling (Figure 5A) is often preferred, as it avoids the need for dual antiplatelet therapy required with stent-assisted coiling. Hypercompliant balloons are ideal for this purpose. An added advantage of balloon remodeling is the ability to temporarily occlude the parent vessel in the event of intraprocedural rupture, providing hemostasis [10].
Stent-assisted coiling can be performed using several techniques. The “jailing” technique (Figure 5B) involves deploying the stent after the aneurysm is catheterized but before coil deployment. Alternatively, the “crossing” technique (Figure 5C) involves deploying a stent across the aneurysm neck first, followed by navigation through the stent struts to access the aneurysm.
Other techniques include coiling with the dual microcatheter technique (Figure 5D), Y-stent configuration for basilar tip aneurysms (Figure 5E), and flow diversion for blister or dorsal carotid wall aneurysms.
The Comaneci device is a retrievable, stent-like expandable device used to prevent coil prolapse during coiling of wide-neck aneurysms. It allows continuous blood flow through the parent artery and is removed after the procedure, eliminating the need for long-term antiplatelet therapy [11, 12]. The primary limitation of the dual microcatheter technique is reduced coil packing density, which may compromise long-term durability due to the absence of adjunctive support from a balloon or stent [13]. Partial coiling can be employed in the acute phase to prevent rebleeding, with definitive treatment deferred until the patient is stabilized [14].
Flow diverters (Figure 5F) are novel endovascular devices designed to redirect blood flow away from the aneurysm sac while preserving flow through side branches. These biocompatible, tightly braided metallic stents (e.g., Pipeline™, Surpass™, Silk Vista™, FRED™, Derivo™, p64/p48™) promote aneurysm thrombosis and eventual obliteration within 3 to 12 months. The efficacy of flow diversion is influenced by metal coverage (typically 30–45%), with Surpass offering the highest coverage. Technological features like DFT (Dual Flat Wire Technology) for visibility, HPC coating for reduced thrombogenicity (e.g., Pipeline Shield, p64 HPC), and devices designed for small-caliber vessels (e.g., Silk Vista Baby, FRED Jr) further refine the approach. Patients must remain on dual antiplatelet therapy for at least 12 months post-procedure. Serial imaging (CTA or DSA) at 3, 6, and 12 months is required to assess aneurysm occlusion and guide antiplatelet management [15].
The pCONus device is a modified stent that facilitates coil placement by reducing metal coverage in the parent vessel while preventing coil herniation [16]. The PulseRider device is a self-expanding nitinol implant with a proximal anchoring segment and distal arches that span the aneurysm neck to support coil placement [17]. The WEB™ device (Figure 5G) is an intrasaccular, self-expanding wire-mesh implant designed to disrupt intra-aneurysmal flow and promote occlusion [18]. The Medina™ Embolization Device (MED) is composed of a spiral core with attached filament leaflets, deployed within the aneurysm sac to disrupt flow [19]. Other available technologies include the Willis® Intracranial Stent Graft System [20], the Contour Device [21], and Neqstent [22].
In a study by Kim et al. [23], 15 patients with wide-neck intracranial aneurysms were treated using either the dual microcatheter technique or stent-assisted coiling. Balloon remodeling was not employed. Of these, 73% underwent embolization with the dual microcatheter technique, and 27% with stent-assisted coiling. Post-procedural angiography showed Raymond-Roy Class II occlusion in 27% of cases.
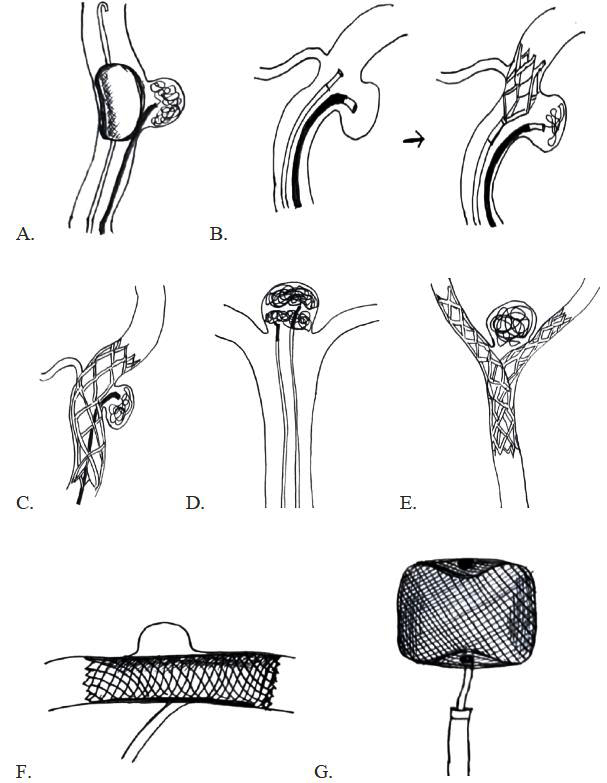
Figure 5: Schematic diagrams of various techniques and devices used in the endovascular management of wide-neck aneurysms: (A) Balloon-assisted coiling; (B) Stent-assisted coiling – Jailing technique; (C) Stent-assisted coiling – Crossing technique; (D) Double microcatheter technique; € Y-configuration stent-assisted coiling of wide-neck bifurcation aneurysm; (F) Flow diverter; (G) Prototype of intra-aneurysmal flow disruptor: Woven Endo-Bridge (WEB) device.
Morsy et al. [24] treated 40 patients with various endovascular methods: simple coiling in 3, dual catheter technique in 5, balloon-assisted coiling (BAC) in 16, stent-assisted coiling (SAC) in 13, and flow diversion (FD) in 3. Immediate angiographic results showed Raymond-Roy Class I occlusion in 90% of patients, and Class II in 2.5%, which required further intervention.
In a study by Sedat et al. [25] the authors have demonstrated that stent-assisted coiling using the LEO stent is an effective treatment option for unruptured wide necked intracranial aneurysms wherein complete occlusion was achieved in 85% cases.
In our study involving 12 patients, balloon-assisted coiling was performed in 2 cases, stent-assisted coiling in 5, dual microcatheter technique in 4, and partial coiling in 1. Post-procedural angiography revealed Raymond-Roy Class II occlusion in 9 patients and Class IIIA in 3 patients. These findings are consistent with previously reported outcomes in the literature.
Conclusions
Wide-neck aneurysms pose significant challenges in achieving complete and durable occlusion. Our experience with endovascular treatment has shown it to be safe and feasible, with the primary goal being aneurysm exclusion to prevent rupture, even when perfect angiographic results are not achievable. In complex cases, "remodeling" to stabilize the aneurysm and reduce re-rupture risk is often a viable approach. The advancement of novel techniques, including flow-modifying devices, promises improved precision and long-term results. Future long-term studies and clinical trials with larger sample sizes are essential to evaluate each technique's strengths and weaknesses for optimal management.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Mascitelli JR. Management of wide-neck aneurysms in 2024: how does one make the best treatment decision when there are so many good options? Journal of neurointerventional surgery. 2024; 16:433–434.
[2] Lazareska M, Aliji V, Stojovska-Jovanovska E, Businovska J, Mircevski V, et al. Endovascular treatment of wide neck aneurysms. Open access Macedonian J Med Sci. 2018; 6:2316.
[3] Lee KS, Zhang JJ, Nguyen V, Han J, Johnson JN, et al. The evolution of intracranial aneurysm treatment techniques and future directions. Neurosurgical Review. 2022; 45:1–25.
[4] Dobrocky T, Matzinger M, Piechowiak EI, Kaesmacher J, Pilgram-Pastor S, et al. Benefit of advanced 3D DSA and MRI/CT fusion in neurovascular pathology. Clinical neuroradiology. 2023; 33:669–676.
[5] Brinjikji W, Cloft HJ, Kallmes DF. Difficult aneurysms for endovascular treatment: overwide or undertall? Am J Neuroradiol. 2009; 30:1513–1517.
[6] Singh R, Vani K, Goel G, Mahajan A. Endovascular treatment of a complex broad neck bifurcation aneurysm at peripheral center by pCONus stent: a new neck bridging device. Indian J Vasc Endovasc Surg. 2018; 5:53–55.
[7] Mordasini P, Walser A, Gralla J, Wiest R, Ozdoba C, et al. Stent placement in the endovascular treatment of intracranial aneurysms. Swiss Med Wkly. 2008; 138:646–654.
[8] Joseph S, Kamble R. Current trends in endovascular management of intracranial aneurysms (including posterior fossa aneurysms and multiple aneurysms). Indian J Radiol Imaging. 2008; 18:256–263.
[9] Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke. 2013; 44:2046–2054.
[10] Fiorella D, Woo HH. Balloon assisted treatment of intracranial aneurysms: the conglomerate coil mass technique. J Neurointerv Surg. 2009; 1:121–131.
[11] Zhang X, Zuo Q, Tang H, Xue G, Yang P, et al. Stent assisted coiling versus non-stent assisted coiling for the management of ruptured intracranial aneurysms: a meta-analysis and systematic review. J Neurointerv Surg. 2019; 11:489–496.
[12] Piotin M, Blanc R. Balloons and stents in the endovascular treatment of cerebral aneurysms: vascular anatomy remodeled. Front Neurol. 2014; 5:41.
[13] Harrigan MR, Deveikis JP. Handbook of cerebrovascular disease and neurointerventional technique. New York: Springer; 2009.
[14] Waldau B, Reavey-Cantwell JF, Lawson MF, Jahshan S, Levy EI, et al. Intentional partial coiling dome protection of complex ruptured cerebral aneurysms prevents acute rebleeding and produces favorable clinical outcomes. Acta Neurochir (Wien). 2012; 154:27–31.
[15] Lv X, Yang H, Liu P, Li Y. Flow-diverter devices in the treatment of intracranial aneurysms: a meta-analysis and systematic review. Neuroradiol J. 2016; 29:66–71.
[16] Krupa K, Brzegowy P, Kucybała I, Łasocha B, Urbanik A, et al. Endovascular embolization of wide-necked bifurcation aneurysms with the use of pCONus device: a systematic review and meta-analysis. Clin Imaging. 2021; 70:81–88.
[17] Zuckerman SL, Eli IM, Morone PJ, Dewan MC, Mocco J. Novel technologies in the treatment of intracranial aneurysms. Neurol Res. 2014; 36:368–382.
[18] Lubicz B, Mine B, Collignon L, Brisbois D, Duckwiler G, et al. WEB device for endovascular treatment of wide-neck bifurcation aneurysms. Am J Neuroradiol. 2013; 34:1209–1214.
[19] Sourour NA, Vande Perre S, Maria FD, Papagiannaki C, Gabrieli J, et al. Medina® embolization device for the treatment of intracranial aneurysms: safety and angiographic effectiveness at 6 months. Neurosurgery. 2018; 82:155–162.
[20] Fang C, Tan HQ, Han HJ, Feng H, Xu JC, et al. Endovascular isolation of intracranial blood blister-like aneurysms with Willis covered stent. J Neurointerv Surg. 2017; 9:963–968.
[21] Akhunbay-Fudge CY, Deniz K, Tyagi AK, Patankar T. Endovascular treatment of wide-necked intracranial aneurysms using the novel contour neurovascular system: a single-center safety and feasibility study. J Neurointerv Surg. 2020; 12:987–992.
[22] Fatania K, Patankar DT. Comprehensive review of the recent advances in devices for endovascular treatment of complex brain aneurysms. Br J Radiol. 2022; 95:20210538.
[23] Kim JW, Park YS. Endovascular treatment of wide-necked intracranial aneurysms: techniques and outcomes in 15 patients. J Korean Neurosurg Soc. 2011; 49:97–102.
[24] Morsy A, Mahmoud M, Abokresha AE, Moussa AA, Abdel-Tawab M, et al. Intracranial wide neck aneurysms: clinical and angiographic outcomes of endovascular management. Egypt J Neurol Psychiatr Neurosurg. 2022; 58:108.
[25] Sedat J, Chau Y, Gaudart J, Sachet M, Beuil S, et al. Stent-assisted coiling of intracranial aneurysms using LEO stents: long-term follow-up in 153 patients. Neuroradiology. 2018; 60:211–219.