Case Report
2024
December
Volume : 12
Issue : 4
A case report on Nocardia cyriacigeorgica in renal transplant patient
Velamuri A, Fatima R, Eswaran SP, Bezawada SR
Pdf Page Numbers :- 323-325
Abhishek Velamuri1,*, Rafath Fatima1, Shiva Priya Eswaran1 and Srinivasa Rao Bezawada2
1Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India
2KIMS Foundation and Research Centre, Minister Road, Secunderabad - 500003, Telangana, India
*Corresponding author: Dr. Velamuri Abhishek, Department of Microbiology, Krishna Institute of Medical Sciences, Minister Road, Secunderabad-500003, Telangana, India. Email: avelamuri@yahoo.com
Received 16 July 2024; Revised 11 September 2024; Accepted 17 September 2024; Published 24 September 2024
Citation: Velamuri A, Fatima R, Eswaran SP, Bezawada SR. A case report on Nocardia cyriacigeorgica in renal transplant patient. J Med Sci Res. 2024; 12(4):323-325. DOI: http://dx.doi.org/10.17727/JMSR.2024/12-60
Copyright: © 2024 Velamuri A et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Nocardiosis is an opportunistic infection caused by Nocardia species (spp). Nocardia species are ubiquitous environmental saprophytes occurring in the soil, organic matter, and water. A 36-year-old, female, renal transplant recipient presented with cutaneous and cerebral symptoms of disseminated Nocardiosis. Prompt diagnosis and treatment of the case resulted in a favourable outcome. Nocardiosis is an important infection in transplant recipients, which should be diagnosed early so that appropriate therapy can be instituted.
Keywords: Nocardia, oppurtunistic infections; renal transplant; solid organ transplant; disseminated nocardiosis
Full Text
Introduction
Opportunistic infections are common in patients receiving immunosuppressive therapy after renal transplantation. Nocardiosis is a life-threatening opportunistic infection, affecting 0.04% to 3.5% of patients after solid organ transplant (SOT) [1]. Nocardia is strictly aerobic, partially acid-fast, branching, often beaded, filamentous, and weakly gram-positive bacteria [2]. Nocardiosis may be localized or disseminated, and the usual modes of transmission are by inhalation or through the cutaneous route. Disseminated nocardiosis is defined as involvement of two noncontiguous sites that may or may not include a pulmonary focus.
Case presentation
Six months post-transplant, a 36-year-old female patient presented with the chief complaint of left parotid swelling radiating in nature to the right side of the neck (only meningeal sign in this case) which lasted for more than 7 days before presentation. Associated symptoms included pain upon chewing food and experienced one episode of generalized tonic clonic seizure associated with up rolling of the eyeballs, tongue bite with bowel and bladder incontinence the day before the admission. She was a known case of hypothyroidism, hypertension, chronic kidney disease secondary to medical renal disease of hypertension. After hemodialysis for 4 years, she received a deceased donor renal transplant in November 2023. Post transplant, her baseline creatinine was maintained at 1.6 to 1.8mg/dL.
This patient was maintained on dual immunosuppressive therapy consisting of tacrolimus and myfortic acid once daily.
Laboratory work up revealed serum creatinine was1.70 (baseline creatinine: 1.6 -1.8 mg/dL), white blood cell counts were 8,500/mm3, Chest X-ray was unremarkable, MRI brain was suggestive of well-defined edematous lesion in left occipital region (Infective focus) (Figure 1).

Figure 1: MRI brain showed edematous lesion in the left occipital area.
Echocardiography was negative for any underlying valvular vegetation. The patient underwent ultrasound guided incision and drainage of 5-8cc of purulent fluid from the right thigh, left parotid, left arm and right abdominal region and the samples were sent to the microbiology department for stain and culture.
Gram stain of the pus sample showed plenty of pus cells and moderate Gram positive thin filamentous branching bacilli morphology suggestive of Nocardia (Figure 2).

Figure 2: Nocardia on Grams staining.
Modified Ziehl Neelsen staining revealed acid-fast thin branching filamentous organisms suggestive of nocardiosis (Figure 3).
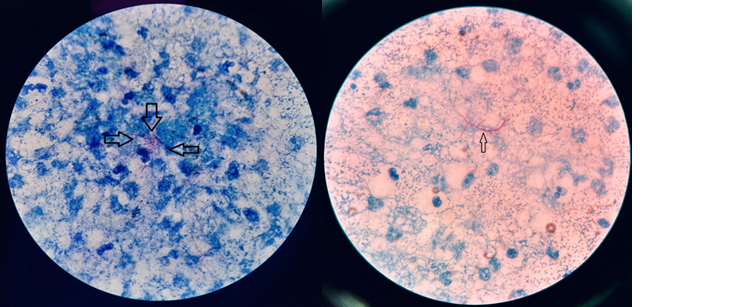
Figure 3: Noccardia on modified ZN staining with 1% H2SO4.
The sample was inoculated on blood agar, chocolate agar, and LJ media. After 48h of incubations chalky, matt, dry, crumbly, colonies grew on plates. Colonies were catalase (positive), urea hydrolysis test (positive), Simmons's citrate (negative). The strain was identified as Nocardia cyriacigeorgica by MALDI-TOF.
The antibiotic sensitivity test was performed by Kirby Bauer disc diffusion method following CLSI guidelines. The isolate was sensitive to cotrimoxazole,amikacin, meropenem, imipenem,ceftriaxone and ciprofloxacin (Figure 4).
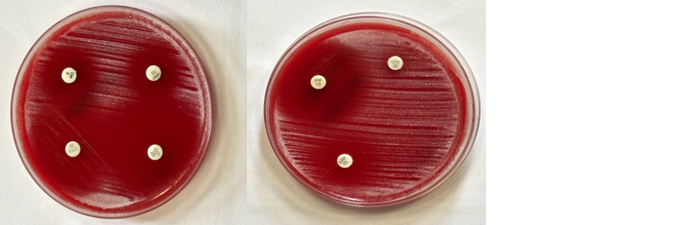
Figure 4: Kirby Bauer disc diffusion for antibiotic susceptibility testing as per CLSI guidelines.
The patient was started on Injection co trimoxazole 80 mg IV three times a day in conjunction with injection cilastatin (500mg) + imipenem (500mg) 500 mg IV twice daily. Tacrolimus and myfortic acid were withheld for two weeks due the underlying infection. The patient's condition gradually improved Figure 5, and she was discharged on IV and oral medications.

Figure 5: Healed suppurative lesion over the inferior aspect of the right thigh.
She was treated with the combination for 6 weeks and was advised to continue cotrimoxazole alone for a duration of 12 months with monthly review visits to the hospital for ruling out possible allograft dysfunction in the future.
Discussion
Renal transplant patients are likely to be at increased risk after immunosuppressive medications for infections [3]. Immunosuppressive therapy leads to compromised T cell and macrophage leading to Nocardia infection [4]. The immunosuppressive agents are initiated to prevent the solid organ transplant rejection in the initial years which if not causes significant raised susceptibility to Nocardiosis [5]. Steroid-sparing protocols with cycloserine, spared the immune compromised patients who underwent renal transplant with marked reduced incidence of around 0.7% [6].
Cutaneous lesions like ulcerations, pyoderma, cellulitis, nodules and subcutaneous abscesses manifest in renal transplant patients with nocardial infections [2, 4, 7] along with hematogenous spread [8]. The patient presented with disseminated nocardiosis which occurs when more than two non-contiguous sites are involved with or without pulmonary system involvement [9].
Standard treatment of choice for nocardiosis is trimethoprim-sulfamethoxazole (TMP- SMX) and is recommended for use in disseminated CNS infection. Some species of nocardia exhibit resistance to TMP-SMX like Nocardia farcinica. Other strains such as Nocardia asteroides and Nocardia nova usually have susceptibility of more than 95% and 89%, respectively. Furthermore, no significant consensus is available on the role of TMP-SMX in Nocardia prophylaxis [10, 11]. Mortality with nocardia infections, post-transplant are not common. A study concluded that the nocardial infection neither caused mortality nor impacted the recipient’s overall survival. Early diagnosis always will suggest a favourable therapeutic outcome [12, 13]. Nocardia cyriacigeorgica has a good antimicrobial susceptibility pattern [14]. The patient clinically improved with TMP SMX and cilastatin + imipenem regimen.
Conclusion
Disseminated nocardiosis with systemic involvement can increase mortality in transplant patients. Early detection and appropriate medical management can result in positive outcomes. Where feasible, speciation of Nocardia should be undertaken to add the knowledge base of prevalent strains.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Coussement J, Lebeaux D, van Delden C, Guillot H, Freund R, et al. European Study Group for Nocardia in Solid Organ Transplantation. Nocardia Infection in Solid Organ Transplant Recipients: A Multicenter European Case-control Study. Clin Infect Dis. 2016; 63:338–345.
[2] Shimizu T, Furumoto H, Asagami C, Kanaya K, Mikami Y, et al. Disseminated subcutaneous Nocardia farcinica abscesses in a nephrotic syndrome patient. J Am Acad Dermatol. 1998; 38:874–876.
[3] Cunha BA. Central nervous system infections in the compromised host: a diagnostic approach. Infect Dis Clin North Am. 2001; 15:567–590.
[4] Wilson JP, Turner HR, Kirchner KA, Chapman SW. Nocardial infections in renal transplant recipients. Medicine (Baltimore). 1989; 68:38–57.
[5] Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore). 2004; 83:300–313.
[6] Arduino RC, Johnson PC, Miranda AG. Nocardiosis in renal transplant recipients undergoing immunosuppression with cyclosporine. Clin Infect Dis. 1993; 16:505–512.
[7] Satterwhite TK, Wallace RJ. Primary cutaneous nocardiosis. JAMA. 1979; 242:333–336.
[8] Arjun R, Padmanabhan A, Attunuru BPR, Gupta P. Disseminated nocardiosis masquerading as metastatic malignancy. Lung India. 2016; 33:434–438.
[9] Anwar S, Acharya S, Thapa K, Adhikari N, Mobarakai N. Brain Abscesses by Nocardia: An Interesting Case. Cureus. 2023;15:e38911.
[10] Michael MM, June MB, Lori CH, Theodore AS. Evaluation of Therapy for Nocardia asteroides Complex Infections. Infect Dis Clin Pract. 1995; 4:287.
[11] Brown-Elliott BA, Biehle J, Conville PS, Cohen S, Saubolle M, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol. 2012; 50:670–672.
[12] Simpson GL, Stinson EB, Egger MJ, Remington JS. Nocardial infections in the immunocompromised host: A detailed study in a defined population. Rev Infect Dis. 1981; 3:492–507.
[13] Boamah H, Puranam P, Sandre RM. Disseminated Nocardia farcinica in an immunocompetent patient. ID Cases. 2016; 6:9–12.
[14] Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microb Review. 2006; 19:259–282.