Orginal Research
2024
September
Volume : 12
Issue : 3
Optic nerve head and retinal nerve fiber layer changes by spectral domain optical coherence tomography in glaucoma suspects at tertiary care hospital
Wagganavar PB, Shreenivasa A, Adappa K, Deshpande KK
Pdf Page Numbers :- 218-222
Pooja B Wagganavar1, Shreenivasa A2,*, Karishma Adappa3 and Kavya Krishnarao Deshpande4
1Department of Ophthalmology, Karwar Institute of Medical Sciences, Karwar, Karnataka 581301, India
2Department of pulmonary medicine, Karwar Institute of Medical Sciences, Karwar, Karnataka 581301, India
3Department of Ophthalmology, BGS Global Institute of Medical Sciences, Bangalore, Karnataka 560060, India
4Department of Ophthalmology, Vydehi Institute Medical Sciences and Research Center, Bangalore, Karnataka 560066, India
*Corresponding author: Dr. Shreenivasa A, Assistant Professor, Department of Pulmonary Medicine, Karwar Institute of Medical Sciences Karwar, Karnataka 581301, India. Email: drshreenivasa240@gmail.com
Received 16 April 2024; Revised 13 June 2024; Accepted 19 June 2024; Published 26 June 2024
Citation: Wagganavar PB, Shreenivasa A, Adappa K, Deshpande KK. Optic nerve head and retinal nerve fiber layer changes by spectral domain optical coherence tomography in glaucoma suspects at tertiary care hospital. J Med Sci Res. 2024; 12(3):218-222. DOI: http://dx.doi.org/10.17727/JMSR.2024/12-41
Copyright: © 2024 Wagganavar PB et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Glaucoma is a progressive optic neuropathy characterized by functional loss or optic nerve dysfunction which is one of the leading causes of irreversible but preventable blindness. The aim of the study was to determine which of the retinal nerve fiber layer and optic nerve head parameters are reliable markers of optic nerve damage in glaucoma suspects.
Methods: Observational cross-sectional study of 250 patient’s including147 glaucoma suspect eyes, 103 normal eyes. All subjects underwent complete eye examination and imaging with the spectral domain optical coherence tomography (SD-OCT) cirrus-TM OCT (Carl Zeiss Meditec, Dublin, CA). Retinal nerve fiber layer (RNFL) and optic nerve head (ONH) OCT protocols were used to evaluate all study participants. The main outcome measures were the difference in OCT parameters among groups.
Results: Study shows statistically significant difference in the average RNFL thickness, superior, nasal, inferior, temporal quadrants, rim area, cup volume, average cup to disc ratio (CDR), vertical CDR with P value <0.001 between the two groups. Average CDR, vertical CDR and cup volume had significantly greater AROC values (ROC: 0.99, 0.98, 0.95) than RNFL parameters for discriminating glaucoma suspects from normal eyes.
Conclusion: Assessment of RNFL, macular and ONH damage with SD-OCT has been proven useful for diagnosing the disease at different levels of severity, as well as for quantifying risk in glaucoma suspects. In our study ONH measurements, as provided by the SD OCT, have more diagnostic value than RNFL parameters in the diagnosis of glaucoma suspects from normal.
Keywords: glaucoma suspects; optic nerve head; retinal nerve fiber layer; optical coherence tomography
Full Text
Introduction
Glaucoma is a leading cause of irreversible but preventable blindness all over the world, the prevalence and Disability adjusted life years (DALY) number of glaucoma increased from 1990 to 2019 globally, has caused a huge economic burden to the family and society [1]. Glaucoma is the second leading cause of permanent blindness in the United States and occurs most often in older adults [2]. Global prevalence of glaucoma is 3.54%. In 2013 number of people with glaucoma worldwide was estimated to be 64.3 million increasing to 76.0 million in 2020 is expected to increase to 112 million people in 2040 due to the rapid increase in global population [3-4]. Being a silent disease of progressive nature is a major health concern. As per the epidemiological studies at least half of glaucoma patients are undetected. In 2020, 43.78 million POAG cases were projected to be undetected, of which 76.7% were in Africa and Asia [5].
Glaucoma is a progressive optic neuropathy characterized by functional loss or optic nerve dysfunction caused by specific and progressive injury to the optic nerve head (ONH) and retinal nerve fiber layer (RNFL), that can lead to permanent loss of peripheral or central vision [6-7]. There occurs significant amount of ganglion cell death (25-30%) before any visual field defect is produced [8-9]. The Changes in the RNFL thickness often precede optic disc damage and glaucomatous visual field loss [10]. Glaucoma is a controllable disease with appropriate screening and treatment and its progress can be arrested before significant effects on visual field can occur. Novel technologies like Spectral Domain Optical Coherence Tomography (SD-OCT) have been developed to find more sensitive ways to detect early glaucoma using structural measurements which provide quantitative and objective assessments of the RNFL and ONH. Thus, OCT is being used as a screening tool in identifying glaucoma suspects in high-risk groups. There is still no clear consensus as to which RNFL or ONH parameter is most reliable for early detection of glaucoma [11]. The purpose of this study is to determine which of the retinal nerve fiber layer and optic nerve head parameters are reliable markers of optic nerve damage in glaucoma suspects.
To evaluate glaucoma suspects by measuring retinal nerve fiber layer and optical nerve head parameters using spectral domain optical coherence tomography and to identify the most sensitive and specific parameter in RNFL and ONH for early detection of glaucoma.
Material and methods
Study design: Observational cross-sectional study. Assuming global prevalence of glaucoma as 3.54%, with an absolute precision of 3%, a sample size of 150 has been calculated (alpha error of 5%). The study population was composed of 147 glaucoma suspect eyes, 103 normal subject eyes who are patients attending to the department of Ophthalmology at tertiary care hospital between January 2021 to December 2022. Informed consent was taken from all participants, and the study complied by the principles of the Declaration of Helsinki. Ethical clearance was obtained from the institution.
The inclusion criteria for patients consist of 30-60 years of age, Both male & female, BCVA 20/40, corrected IOP >10 mmHg (Normotensive or hypertensive), spherical refraction < -3D and cylindrical <-2D, an open angle on gonioscopy and a normal visual field, patients having any one or more of following disc features suspicious of glaucomatous optic neuropathy i.e. cup/ disc ratio>0.5, asymmetry cupping of optic disc >0.2, neuro retinal rim thinning, superior and inferior notching of rim, hemorrhages on the disc, RNFL defect.
Exclusion criteria: The subjects were excluded if they had a corneal or lenticular opacity, cataract nuclear sclerosis grade 3& 4(LOCS III scale), angle closure glaucoma, diagnosed cases of glaucoma, inflammation like uveitis, trauma, steroid induced glaucoma, congenital and hereditary optic nerve and retinal disorders, primary and consecutive optic atrophy, uncontrolled DM and HTN with retinopathy changes, unreliable visual fields, if the OCT images were of poor quality.
Method of data collection
The subjects were identified as a glaucoma suspect eye based on the presence of optic nerve findings suspicious for glaucoma with normal or ocular hypertensives with normal visual fields at the time of the OCT imaging session [12].
The subjects were categorized as normal eyes having an intraocular pressure of 20 MmHg or less on at least three different days, normal optic disc, normal visual fields, open angle on gonioscopy. All subjects underwent detailed ocular examinations including, best corrected visual acuity, slit lamp examination, fundoscopy, optic disc photography, intraocular pressure recording, gonioscopy, central corneal thickness. Retinal nerve fiber layer and optic nerve head parameters were evaluated by using spectral domain optical coherence tomography (Software version 6.5.0.772) and visual field evaluation was done using automated perimetry, humphrey field analyzer model 750i (Zeiss Humphrey Systems, Dublin, CA), by using program 30-2, Swedish interactive threshold algorithm standard strategy. At least 2 reliable standard automated perimetry were performed to minimize the learning effect.
Scanning protocols: Optic nerve head analysis and retinal nerve fiber analysis using spectral domain, optical coherence tomography - carl zeiss, cirrus operator.
Spectral-domain OCT imaging was performed with the CirrusTM high-definition OCT (model 4000-11970 software version 6.5.0.772. produced by Carl Zeiss Meditech, Inc.). An optic disc cube scan protocol was used to measure the RNFL thickness in a 6×6mm2 area consisting of 200×200 axial scans (pixels) at the optic disc region. The RNFL thickness at each pixel was measured, and an RNFL thickness map was generated. A calculation circle of 3.46 mm in diameter consisting of 256 A-scans then was positioned automatically around optic disc. RNFL thicknesses (Average, superior, nasal, inferior and temporal RNFL thickness) and ONH parameters (Rim area, disc area, cup volume, average CDR, vertical CDR) were analyzed. All the OCT scans included in the study had a signal strength of >6. Saccadic eye movement was detected with the line-scanning ophthalmoscope overlaid with OCT en face during OCT imaging. Images with motion artifacts were rescanned at the same visit.
Statistical methods
Students t-test used to find the significance difference between the age, intra ocular pressure, RNFL thickness and ONH parameters with Groups (Glaucoma suspects and normal) and expressed as mean and standard deviation. Chi square test was used to measure the association between the age groups, gender, co-morbidity. The statistical analysis was performed by STATA 11.2 (College Station TX USA). Receiver operating characteristics curve (ROC) used for predicting the best cut off values from RNFL thickness and ONH parameters for glaucoma suspect cases. Area under receiver operating characteristics (ROC) measures test’s diagnostic ability, that is, its power to correctly classify those with and without the disease.
Results
Study includes 250 patients among which 147 identified as glaucoma suspects and 103 normal eyes. Mean age of glaucoma suspects is 44.20 ± 8.11 and of normal subjects is 45.92 ± 8.68. The majority of the patients are in the age group of 40-50 years. The average IOP in the patients with glaucoma suspects was significantly higher (P value of <0.001) 15.59 ± 2.89 mmHg than in the normal group 13.49 ± 1.74 mmHg Mean ± SD.
Table 1: Values of SD OCT RNFL and ONH parameters.
|
Parameters
|
Glaucoma Suspects
|
Normal
|
p value
|
|
RNFL thickness (µm)
|
Mean ± SD
|
Mean ± SD
|
|
Average RNFL
|
77.09 ± 19.25
|
95.50 ± 7.88
|
<0.001
|
|
Superior RNFL
|
95.50 ± 35.61
|
117.62±15.11
|
<0.001
|
|
Nasal RNFL
|
61.35 ± 17.64
|
74.05 ± 12.66
|
<0.001
|
|
Inferior RNFL
|
95.22 ± 32.65
|
123.67±15.05
|
<0.001
|
|
Temporal
|
58.45 ± 12.60
|
64.75 ± 8.17
|
<0.001
|
|
ONH parameters
|
|
Rim area (mm2)
|
1.15 ± 0.31
|
1.53 ± 0.41
|
<0.001
|
|
Disc area (mm2)
|
2.31 ± 0.50
|
2.19 ± 0.34
|
0.37
|
|
Cup volume (mm3)
|
0.55 ± 0.23
|
0.17 ± 0.12
|
<0.001
|
|
Average CDR
|
0.71 ± 0.07
|
0.43 ± 0.09
|
<0.001
|
|
Vertical CDR
|
0.68 ± 0.09
|
0.43 ± 0.09
|
<0.001
|
Significant thinning of RNFL in all 4 quadrants and 360 degrees of optic disc, among ONH parameters significant reduction of mean Rim area noted, whereas mean value of cup volume, average CDR, and vertical CDR is significantly increased (P < 0.001) in newly detected glaucoma suspects compared to normal subjects. However, two groups did not show any difference in the disc area, p value 0.37.
Table 2: Diagnostic accuracy of SD-OCT RNFL and ONH parameters to discriminate between normal and glaucoma suspects.
|
Parameters
|
ROC
|
Cutoff
|
Sensitivity
|
Specificity
|
|
Average RNFL
|
0.83
|
87
|
90.29%
|
66.31%
|
|
Superior RNFL
|
0.69
|
110
|
68.93%
|
58.50%
|
|
Nasal RNFL
|
0.70
|
67
|
76.70%
|
51.02%
|
|
Inferior RNFL
|
0.80
|
116
|
70.87%
|
70.75%
|
|
Temporal RNFL
|
0.66
|
62
|
60.19%
|
59.86%
|
|
ONH Parameters
|
|
DISC AREA
|
0.58
|
2.15
|
62.59%
|
44.46%
|
|
CUP Volume
|
0.95
|
0.30
|
85.71%
|
89.32%
|
|
Average CDR
|
0.99
|
0.55
|
95.92%
|
91.26%
|
|
Vertical CDR
|
0.98
|
0.54
|
94.56%
|
83.50%
|
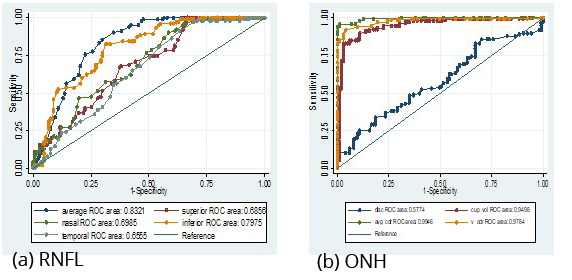
Figure 1a,b: Showing area under receiver operating curves for RNFL and ONH parameters.
In table 2 and Figure 1 (AROC): Average RNFL thickness followed by inferior RNFL thickness have high ROC values 0.83, 0.80 with 90.29%, 70.87% sensitivity and 66.31%, 70.75% specificity respectively. Whereas Nasal RNFL thickness, superior RNFL thickness, temporal RNFL thickness have ROC values 0.70, 0.69 and 0.66. with 76.70%, 68.93%, 60.19% sensitivity and 51.02%, 58.50%, 59.86% specificity respectively. Among optic disc parameters average CDR followed by vertical CDR and cup volume having greater ROC areas (ROC: 0.99, 0.98, 0.95 respectively) with sensitivity of 95.92%, 94.56%, 85.71% and specificity 91.26%, 89.32%, 83.50% respectively. Whereas disc area has low ROC value (0.58) with less sensitivity 62.59% and less specificity 44.46% and rim area with very low ROC (<0.5) have very poor predictability.
Discussion
Glaucoma is a progressive degeneration of the optic nerve, with loss of retinal ganglion cells, thinning of the retinal nerve fiber layer, and increasing excavation of the optic disc. Damage to ganglion cells can occur via various mechanisms including baric trauma, ischemia and impact of metabolic toxins, which triggers an inflammatory process and secondary degeneration in the ONH causing irreversible blindness [6-9]. Early detection and monitoring are critical to the diagnosis and management of glaucoma, and preservation of vision and quality of life [13]. The evaluation of glaucoma is highly dependent on the functional assessment of a patient’s vision and the structural assessment of the retina and optic nerve [14].
Optical coherence tomography (OCT) has become a commonly utilized imaging modality that aids in the detection and monitoring of structural glaucomatous damage, owing to its ability to visualize the retinal substructure. Study shows statistically significant difference in the average RNFL thickness, in the superior, nasal, inferior, temporal quadrants of optic nerve head, in the rim area, cup volume, average CDR, vertical CDR with p value <0.001 between the two groups. However, two groups did not show any difference in the disc area, (p value 0.37). To detect the most sensitive OCT parameters that can differentiate between glaucoma suspect eyes from normal eyes, Receiver operating characteristics curve (ROC) were calculated which provides cut off value with sensitivity and specificity of the parameter. ROC of 1 (100% sensitivity and 100% specificity) represents a perfect test, whereas an ROC of 0.5 indicates a completely worthless test. Larger the ROC value more is the diagnostic accuracy. the best optic disc parameters to discriminate between glaucoma suspects from normal eyes found to be average CDR followed by vertical CDR and cup volume with largest ROC areas (ROC: 0.99, 0.98, 0.95 respectively).
The best RNFL parameters to discriminate between two groups found to be average RNFL thickness followed by inferior RNFL thickness with high ROC values 0.83, 0.80. Overall, the best parameters among ONH & RNFL found to be average CDR (0.99) followed by vertical CDR (0.98) and cup volume (0.95), average RNFL thickness (ROC 0.83) followed by inferior RNFL thickness (0.80). Hence average CDR, vertical CDR and cup volumes are most sensitive and specific parameters for early detection of glaucoma from normal eyes. however, disc area and RIM areas are poor predictors. Studies have reported good diagnostic capability of ONH parameters (disc area, rim area, cup-to-disc ratio, cup volume, RNFL thickness) and macular parameters. [15-17]. Macular thickness had high discriminating power comparable with peripapillary RNFL thickness parameters [18]. Recent reports of the utility of macular Ganglion cell complex analysis for advanced glaucoma [19]. Evidence for the relative performance of ONH parameters has been conflicting. Some studies have demonstrated the superior performance of RNFL thickness compared with other ONH parameters, especially for detecting pre-perimetric glaucoma [17, 19] Chen et al, reported the best evidence-based parameters for detecting glaucomatous nerve damage were RNFL thickness, GCIPL thickness, rim area, and vertical cup-to-disc ratio [20]. Saha M e.t.al found average RNFL thickness of 0.99 AROC [18].
Study in which most of the RNFL parameters demonstrated statistically significant difference and vertical CDR, average CDR and rim area with ROC values (8.93, 0.891, 0.841 respectively) had better diagnostic precision in differentiating between pre-perimetric glaucoma and normal eyes [11]. Artificial intelligence -Deep learning model (AUC of 0.938) had shown the probability of glaucoma progression from SD-OCT measurements of the RNFL thickness [21]. Thus, in our study ONH parameters are most sensitive in detecting glaucoma suspects from normal compared to RNFL parameters. The ability to detect and quantify structural damage is essential for proper diagnosis and management of glaucoma. Our results are in agreement with study in which ONH parameters had better diagnostic precision in identification of glaucoma suspect eyes. Whereas RNFL parameters were better at discriminating between normal, developed & terminal glaucoma [10]. Study by Kaushik S, et al reported there was no difference in average RNFL thickness OR Ganglion cell analysis to discriminate between normal controls and glaucoma suspects. However average RNFL thickness had significantly greater AROC values than average GCA for discriminating glaucoma suspects (both suspicious discs and OHT) from glaucoma [p = 0.03 and 0.05, respectively) [22]. Assessment of RNFL, macular and ONH damage with SDOCT has been proven useful for diagnosing the disease at different levels of severity, as well as for quantifying risk in glaucoma suspects.
Limitations: No follow-ups done in our study to look for the progression of disease and impact on OCT parameters. Multicentric with large sample size required in future studies.
Conclusion
Early diagnosis and detection of disease progression are critical in glaucoma, the leading cause of irreversible blindness worldwide. The use of non- invasive imaging modalities such as OCT, with its advanced technology allows enhanced image quality, increased scan speed, in vivo examination of the key structures of glaucomatous changes of the retina contributing to promising tool in diagnosis of glaucoma suspects. In our study ONH parameters had best ability to detect early damage of glaucoma compared to RNFL parameters, hence to discriminate glaucoma suspects from normal group. These results reinforce the importance of optic disc and RNFL examination and monitoring in glaucoma suspect patients in preventing irreversible nerve fiber loss and visual field loss.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Lin Y, Jiang B, Cai Y, Luo W, Zhu X, et al. The Global Burden of Glaucoma: Findings from the Global Burden of Disease 2019 Study and Predictions by Bayesian Age-Period-Cohort Analysis. J Clin Med. 2023; 12:1828.
[2] Ezinne NE, Shittu O, Ekemiri KK, Kwarteng MA, Tagoh S, Ogbonna G, Mashige KP. Visual Impairment and Blindness among Patients at Nigeria Army Eye Centre, Bonny Cantonment Lagos, Nigeria. Healthcare (Basel). 2022; 10:2312.
[3] Tham YC, Li X, Wong TY, Quigley HA, Aung T, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmol. 2014; 121:2081–2090.
[4] Kang J.M., Tanna A.P. Glaucoma. Med Clin North Am. 2021; 105:493–510.
[5] Soh Z, Yu M, Betzler BK, Majithia S, Thakur S, Tham YC, Wong TY, Aung T, Friedman DS, Cheng CY. The Global Extent of Undetected Glaucoma in Adults: A Systematic Review and Meta-analysis. Ophthalmol. 2021; 128:1393–1404.
[6] Stein J.D., Khawaja A.P., Weizer J.S. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA. 2021; 325:164–174.
[7] Schuster AK, Erb C, Hoffmann EM, Dietlein T, et al; The Diagnosis and Treatment of Glaucoma. Dtsch Arztebl Int. 2020; 117:225–234.
[8] Evangelho, K., Mogilevskaya, M., Losada-Barragan, M. et al. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmol. 2019; 39:259–271.
[9] Mwanza JC, Budenz DL. Optical coherence tomography platforms and parameters for glaucoma diagnosis and progression. Curr Opin Ophthalmol. 2016; 27:102–110.
[10] Kasumovic S, Pavljasevic S, Cabric E, Mavija M, Daciclepara S, Jankov M. Correlation between retinal nerve fiber layer and disc parameters in glaucoma suspected eyes. Med Arch. 2014; 68:113–116.
[11] Pomorska M, Berkowska P, Hojlo M, Pytrus H, Grazybowski A. Application of optical coherence tomography in glaucoma suspect eyes. Clin Exper Optomet. 2012; 95:78–88.
[12] Gedde SJ, Lind JT, Wright MM, Chen PP, Muir KW, Vinod K, Li T, Mansberger SL; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern®. Ophthalmol. 2021; 128:P151–P192.
[13] Geevarghese A, Wollstein G, Ishikawa H, Schuman JS. Optical Coherence Tomography and Glaucoma. Annu Rev Vis Sci. 2021; 7:693–726.
[14] Boland MV, Quigley HA. Evaluation of a combined index of optic nerve structure and function for glaucoma diagnosis. BMC Ophthalmol. 2011; 11:6.
[15] Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014; 98:ii15–19.
[16] Lisboa R, Paranhos A Jr., Weinreb RN, Zangwill LM, et al. Comparison of different spectral domain oct scanning protocols for diagnosing preperimetric glaucoma. Investig Ophthalmol Vis Sci. 2013; 54:3417–3425.
[17] Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2013; 54:4422–4429.
[18] Saha M, Bandyopadhyay S, Das D, Ghosh S. Comparative analysis of macular and peripapillary retinal nerve fiber layer thickness in normal, glaucoma suspect and glaucomatous eyes by optical coherence tomography. Nepal J Ophthalmol. 2016; 8:110–118.
[19] Sung KR, Na JH, Lee Y. Glaucoma diagnostic capabilities of optic nerve head parameters as determined by Cirrus HD optical coherence tomography. J Glaucoma. 2012; 21:498–504.
[20] Chen TC, Hoguet A, Junk AK, Nouri-Mahdavi K, Radhakrishnan S, Takusagawa HL, Chen PP. Spectral-Domain OCT: Helping the Clinician Diagnose Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmol. 2018; 125:1817–1827.
[21] Mariottoni EB, Datta S, Shigueoka LS, Jammal AA, Tavares IM, Henao R, Carin L, Medeiros FA. Deep Learning-Assisted Detection of Glaucoma Progression in Spectral-Domain OCT. Ophthalmol Glaucoma. 2023; 6:228–238.
[22] Kaushik S, Kataria P, Jain V, Joshi G, Raj S, Pandav SS. Evaluation of macular ganglion cell analysis compared to retinal nerve fiber layer thickness for preperimetric glaucoma diagnosis. Indian J Ophthalmol. 2018; 66:511–516.