Full Text
Introduction
Paralytic ileus refers to absence of bowel movement and inability to tolerate oral intake from non-mechanical causes that disrupt the normal coordinated propulsive activity of the gastrointestinal tract [1, 2].
It is widely believed that normal ileus in postoperative state lasts 0 to 24 hours in small intestine; 24 to 48 hours in stomach and 48 to 72 hours in the colon [1]. Various end points have been used to measure the recovery from ileus–such as passage of flatus, return of normal bowel sounds, tolerance of solid food and radiological emptying studies. However, there is no consensus on which of these parameters is most accurate and the time of recovery from ileus depends to a large extent on the type of surgery done and the end point used. It is generally agreed that gastric and small intestinal activity return within a few hours of surgery and colonic activity a day or two thereafter.
Some degree of postoperative ileus is normal after all abdominal and some non-abdominal surgeries, but it resolves within 4-5 days. It becomes a cause for concern when the ileus becomes unduly prolonged. Apart from the prolonged hospital stay and the consequent increase in the costs of health care resources- delayed enteral nutrition, delayed ambulation resulting in atelectasis, deep vein thrombosis etc and rarely perforation are the additional serious complications. Ileus usually occurs throughout the gastrointestinal tract but occasionally only a part of the gut is involved localized ileus. Post-operative ileus is multifactorial in origin and determining the etiology and understanding the pathophysiological mechanisms are essential to provide rational treatment for its correction.
The present review that is confined to paralytic ileus occurring after abdominal surgery, initially describes the normal neural anatomy and physiology of the gut, the aetiology and various pathogenic pathways of ileus, followed by diagnosis and current status of various elements of treatment and ends with a management algorithm.
Physiology
Normal GI motility is a coordinated process of the electrophysiological activity of the smooth muscle cells, neural input from the intrinsic and autonomous nervous systems, hormonal interactions and the integrated smooth muscle contraction. The neural networks that influence the gut include the intrinsic and the extrinsic systems (Figure 1).
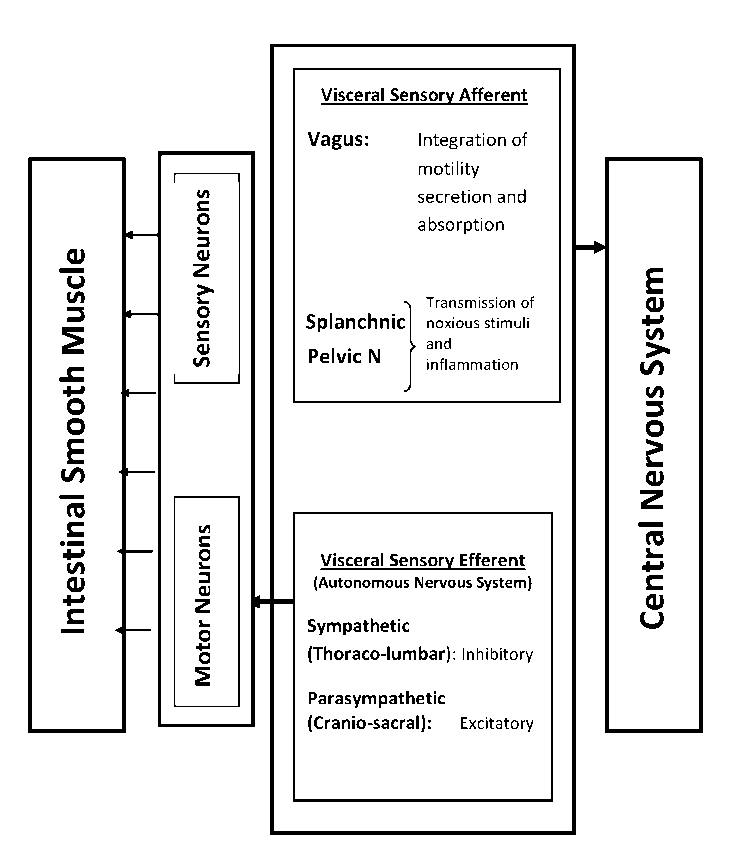
Figure 1: Neural networks of gastrointestinal tract.
Intrinsic network
The neurons located in the intestinal wall comprise the intrinsic neural network and is also called the enteric nervous system (ENS). It consists of two ganglionated plexuses: the outer, larger myenteric (Auerbach’s) plexus lying between the longitudinal and circular layers of the gut and the inner, smaller submucosal (Meissner’s) plexus. Stimulation of the myenteric plexus increases the tone of the gut wall, intensity and rhythm of contractions and conduction velocity. The submucosal plexus mainly controls local secretary and absorptive activities.
Extrinsic network
The extrinsic neural network comprises
a. Visceral sensory afferents
a. Vagus nerve
b. Splanchnic nerves
c. Pelvic nerves
b. Visceral motor efferents
a. Sympathetic thoraco-lumbar outflow
b. Parasympathetic cranio-sacral outflow
Vagal sensory afferents are important in the coordinated integration of motility, secretion and absorption while spinal afferents (splanchnic and pelvic nerves) are responsible for the transmission of noxious stimuli and inflammation. On the other hand, Motor sympathetic activity is generally inhibitory to both GI secretion and motor activity and causes contraction of sphincters and blood vessels while parasympathetic is excitatory to motor activity and causes relaxation of sphincters and vascular smooth muscle.
Aetio-pathogenesis
The commonest causes of postoperative ileus in clinical practice are:
1. Sepsis
2. Intra-peritoneal inflammation, collections, abscesses
3. Retroperitoneal haematoma
4. Alterations in chemical composition of internal environment
a. Deficiency of Na+, Cl-, K+, Mg++
b. Anaemia
c. Hypoosmolality
d. Hypoproteinemia
e. Uraemia
5. Hypothyroid state
6. Mesenteric vascular insufficiency
7. Drugs
a. Morphine
b. Diphenoxylate
c. Antidepressant drugs
d. Antiparkinsonian drugs
e. Antacids
f. anticoagulants
Three common pathways have been identified in the pathogenesis of postoperative ileus which could be associated with many clinical conditions. These include neurogenic inhibitory reflexes, inflammatory and pharmacologic pathways.
Neurogenic inhibitory reflexes
Neurogenic bowel inhibition is predominantly through pain-induced neural reflexes mediated through sympathetic hyperactivity [1]. Pain-induced reflexes also contribute to GI inhibition through the production of endogenous opioids, nitric oxide, corticotrophin releasing hormone, somatostatin and glucagon. The clinical application of this mechanism is the use of laparoscopic surgery and blockade of spinal afferents with epidural local anaesthetics [2]. Ileus may still occur after laparoscopic surgery in spite of less pain and manipulation of the bowel possibly because of the opioid analgesics, mediated by the stimulation of GI opioid receptors by exogenous opioids.
Inflammatory pathway
Surgical manipulation, rough handling and trauma result in an inflammatory response that inhibits the smooth muscle of the gastrointestinal tract [3]. This is mediated by local macrophages and mast cells [4]. Similarly toxic material resulting from localized collections may also manifest as localized ileus. Bowel wall oedema from fluid overload, inflammation or hypoproteinemia is another mechanism of reducing the intestinal motility. The resulting electrolyte abnormalities from fluid shifts and third space losses aggravate the problem.
The inflammatory and pharmacologic pathways are interrelated as activated inflammatory cells liberate a variety of humoral substances that further increase the inhibition of intestinal motility by increasing the inflammatory cell recruitment.
Pharmacological pathway
The pharmacologic deterrents to intestinal motility are through various inhibitory neurotransmitters as nitric oxide, vasoactive intestinal polypeptide (VIP), substance P, calcitonin gene- related peptide, corticotrophin releasing factor (CRF) and decreased motilin, a motor stimulatory hormone.
Opioid classes of drugs are extremely effective analgesics. Unfortunately, they have well-known inhibitory effects on GI motility too. They decrease GI secretion, gastric motility and emptying and decrease propulsive colonic movements. They also cause increase in the resting tone and small intestinal periodic spasms. They act by stimulation of opioid receptors in the bowel and are mediated at the level of enteric nervous system. The blockage occurs through predominantly three types of receptors: µ, δ and К with several subtypes. The effects of opioids on GI motility are mediated through µ2 receptors. One of the novel treatment modalities of postoperative ileus is by selectively blocking these receptors [5]. This mechanism is also aggravated in patients with analgesic tolerance; all suggesting that postoperative opioid management is an important determinant of postoperative ileus.
Many of the anaesthetic agents are known to impair GI motility but seldom cause prolonged ileus unless an opioid is used as the analgesic component [5, 6]. The fact that ileus is not a general accompaniment of non-abdominal surgery also discounts the role of anaesthesia in postoperative ileus.
Diagnosis
It is critically important to differentiate physiological from pathological causes of postoperative ileus as also from mechanical intestinal obstruction (Table 1).
Table 1: Functional intestinal obstructions compared to mechanical obstruction
| |
Ileus
|
Pseudo-obstruction
|
Mechanical Obstruction (Simple)
|
|
Symptoms
|
Mild abdominal pain, bloating, nausea, vomiting, obstipation, constipation
|
Colicky abdominal pain, constipation, obstipation, nausea, vomiting, anorexia
|
Colicky abdominal pain, constipation, obstipation, nausea, bilious or faeculent vomiting, anorexia
|
|
Physical Examination Findings
|
Silent abdomen, distension, tympanic
|
Borborygmi, tympanic, peristaltic waves, hypoactive or hyperactive bowel sounds, distension, localized tenderness
|
Borborygmi, peristaltic waves, high-pitched bowel sounds, rushes, distension, localized tenderness
|
|
Plain Radiographs
|
Large and small bowel dilatation, diaphragm elevated
|
Isolated large bowel dilatation, diaphragm elevated
|
Multiple small bowel loops in ladder pattern, paucity of colonic gas distal to lesion, diaphragm mildly elevated, air-fluid levels
|
The factors that need to be taken in to consideration while distinguishing the physiological from pathological causes are: the nature of surgery, intraoperative complications and medical comorbidities. In the absence of mechanical intestinal obstruction, it is generally agreed that a patient has prolonged postoperative ileus if the following features persist for more than five days after open surgery and 3 days after laparoscopic surgery [7]:
• not tolerating oral diet,
• vomiting,
• inability to pass flatus,
• diffuse abdominal discomfort and a feeling of distension confirmed by physical examination
• mild degree of abdominal tenderness due to intestinal stretch and
• sparse bowel sounds.
Clinical evaluation is done to exclude treatable medical and surgical causes (Table 2). History is reviewed for history of diabetes, previous surgeries and medications received. Acute colicky pain with faeculent vomiting would suggest a mechanical cause and presence of fever, tachycardia along with peritoneal signs suggest peritoneal inflammation that may require a percutaneous or surgical intervention.
Table 2: Treatable causes of postoperative ileus
|
Hypokalemia
|
|
Hypomagnesemia
|
|
Uraemia
|
|
Sepsis
|
|
Intraabdominal collections
|
|
Haemoperitoneum
|
|
Anastomotic leaks
|
|
Pancreatitis
|
|
Drugs: Opiates, Anticholinergic drugs, Antihistamines
|
|
Retroperitoneal haemorrhage/collection
|
Laboratory tests should include complete blood picture, serum electrolytes and magnesium, blood urea and creatinine and liver function tests with serum amylase and lipase. Haematocrit would indicate the state of hydration and leucocytosis may indicate sepsis or bowel ischemia whereas liver function tests, amylase and lipase are done to exclude gallstones and pancreatitis.
Plain abdominal radiograph may reveal a panintestinal dilatation including colon with a spotty gas pattern throughout in ileus whereas paucity of gas in colon and multiple air-fluid levels would suggest small bowel obstruction. However, the reported specificity for abdominal radiograph is only 19-43% and is not prognostic for reoperation [8, 9]. Nowadays CT scan of the abdomen with oral Gastrografin is considered gold standard for diagnosing postoperative ileus because it can differentiate early postoperative small intestinal obstruction more than 98% of the time [8]. The sensitivity and specificity are 100% each compared to 19 and 100% respectively for combined clinical evaluation and plain abdominal radiography [8]. CT in addition, has the advantage of identifying other causes of persistent ileus as intraperitoneal collections, anastomotic leakage, perianastomotic phlegmon, haematoma, pancreatitis etc. as also other causes of obstruction as fascial dehiscence and herniations.
Management
Current management of prolonged ileus is mainly directed at prevention of the problem by minimizing the precipitating factors because active treatment has largely been unsatisfactory unless an aetiological cause has been identified and addressed.
Traditionally, the treatment of postoperative ileus revolved around delayed oral feeds, nasogastric decompression, intravenous fluids, correction of electrolyte disturbances and observation. Multimodal fast track programmes that include use of minimally invasive surgical techniques, use of epidural anaesthesia, early mobilization, early enteral feedings and the use of prokinetic agents have been in many studies shown to decrease the length of hospital stay following a variety of abdominal and pelvic surgeries and reduce the postoperative morbidity including the incidence of ileus [8-10]. Most of these measures are standard postoperative treatment modalities but none of them individually has been proved to have a definite role. The current status of these and other strategies is discussed in the following section and an algorithm is suggested based on the strengths of evidence.
Timing of oral feeds
The traditional assumption that early feeding after abdominal surgery aggravates ileus and prolongs the return of normal bowel movements has been disproved in many studies. A Cochrane systematic review [8] found that early postoperative feeding was associated with a faster return of bowel movements in six randomized controlled trials. Similarly a metaanalysis of RCTs [8] of early feeding compared to NPO after gastrointestinal operations found a significantly reduced length of hospital stay after early feeding although the incidence of vomiting increased. Prolonged delay in enteral feeding on the other hand is known to cause mucosal atrophy with subsequent potentially lethal, septic complications as a result of increased bacterial translocation due to decreased gut barrier immunity [8]. Although many trials have demonstrated the feasibility of early oral intake following abdominal surgery and its beneficial effect in reducing the postoperative hospital stay and morbidity, its effect on postoperative ileus is only modest. This is possibly because of the other uncontrolled factors listed above that may aggravate ileus. Nevertheless, since most studies showed that early feeding does not increase the incidence of complications, the most prudent practice is to initiate oral intake as soon as a patient tolerates.
Nasogastric decompression
Nasogastric tube decompression has been a tradition in the postoperative phase of all abdominal operations. It was believed that nasogastric tube shortened postoperative ileus, protected anastomoses and reduced aspiration and the consequent pulmonary problems. However, the opposite has been proven in many studies. A Cochrane review [7] examined 28 randomized controlled trials comparing patients who had routine nasogastric decompression with those, who did not. Interestingly, those with routine NG tube use had significantly slower return of bowel movement, increased pulmonary complications, discomfort and longer hospital stay. This compelling data has resulted in more selective use of NG tube and currently it is used only for recurrent vomiting and symptomatic relief of abdominal bloating.
Minimally invasive surgery
Minimally invasive surgery is immensely popular nowadays because of reduced postoperative pain, less need for powerful analgesia, improved pulmonary function and a shorter hospital stay. A substantial portion of the hospital stay is attributed to the time it takes for ileus to resolve. A recent meta-analysis of short term outcomes of laparoscopic colonic resections including 12 prospective randomized trials with 2512 patients showed that among other benefits, the time to first passage of gas is 33.5% shorter [9]. In fact, ileus after laparoscopic surgery is an ominous sign and should raise the suspicion of occult visceral injuries or mechanical obstruction at trocar sites. The exact mechanism by which laparoscopic surgery reduces the length of postoperative ileus is not clear. It is yet to be determined whether the perceived reduction in the incidence of ileus is a result of reduction of the inflammatory response, reduction of postoperative pain and analgesic use or a combination of various other factors.
Epidural anaesthesia with local anaesthetics
Use of epidural anaesthesia for preventing and treating postoperative ileus is known for a long time [8]. The administration of local anaesthetic agents in the epidural space blocks both the nociceptive afferent signals from the surgical site and inhibitory sympathetic efferent outflow. The chemical sympathectomy that results also eliminates the sympathetic tone of the splanchnic vascular bed causing an increase in blood flow. However, for a good effect, the anaesthetic agent has to be delivered at or above midthoracic level because the epidural space has smaller volume cranially and a given volume of the anaesthetic administered at this level will block more spinal segments than when delivered more caudally. A Cochrane systematic review of 22 randomized trials comparing epidural local anaesthetics to opioid analgesia in abdominal surgery showed that the group receiving epidural local anaesthetics had a significantly reduced mean time for return of GI function (24 versus 37 hours) as defined by passing of flatus or time to first bowel movement [10] Epidural anaesthesia is currently considered one of the more effective modalities to reduce the duration of postoperative ileus.
Early mobilization
Traditionally early ambulation is advocated as a way to reduce postoperative complications. Although it has not been proven to have a statistically significant benefit on GI motility or faster resolution of postoperative ileus, early mobilization is recommended for its other benefits in reducing the incidence of thromboembolism and pulmonary complications.
Surgical technique
Meticulous technique, minimal manipulation and tissue handling are known to reduce postoperative GI dysmotility [10] and should be practiced to reduce the incidence of postoperative ileus.
Analgesia
Opioids are the known GI inhibitors and in spite of their good analgesic effect are best avoided in routine postoperative analgesia protocols. Nonsteroidal anti-inflammatory agents could be good alternatives. They also appear to target one of the key pathways in the pathogenesis of postoperative ileus providing an additional therapeutic benefit
Pharmacological therapy
Numerous drugs have been suggested that include a variety of prokinetic agents, laxatives, NSAIDS, GI hormone analogues and peripheral opioid receptor antagonists. In general they have been disappointing except some of the peripheral opioid receptor antagonists.
Neostigmine, a reversible acetylcholinesterase inhibitor, although was shown to be effective in colonic pseudoobstruction [8] and postoperative ileus following spinal surgery [9] has not been found to have any effect on postoperative ileus in general. The severity of systemic adverse effects however precludes its routine usage.
Multiple trials have not shown a benefit of Metoclopramide [12], Cisapride [12], Erythromycin [14], Ceruletide [14] or laxatives have not shown any significant benefit.
Potentially effective medications
• NSAIDS, as mentioned above, by their duel effects of analgesic-anti-inflammatory property and by targeting one of the known ileus pathways could be potentially beneficial. They also help to reduce the opiate use significantly. However the risk of postoperative GI and surgical site bleeding is increased due to the antiplatelet effect.
• Recently, specific COX-2 inhibitors that lack the antiplatelet effect have been shown to have a beneficial effect on postoperative ileus [12].
• Alvimopan, a novel peripheral opioid receptor antagonist is shown to hasten postoperative GI recovery after bowel surgery [12]. However, its clinical benefit still needs to be confirmed. Methylnaltrexone, another opioid antagonist is also under investigation.
• Atilmotin, a human motilin analogue has been demonstrated to have increased duodenal motility and a faster recovery of the MMC in canine models [10]. A human trial is forthcoming.
Multi-modal fast track programmes
In view of the multifactorial pathophysiology of postoperative ileus, combination of a wide variety of treatment modalities is likely to have a synergistic outcome. This has been proved in multimodal fast-track programmes particularly in colonic surgery [12] and after hysterectomy [13]. Most of these programmes include minimally invasive surgical techniques, epidural anaesthesia and analgesia, optimal pain control, and intensive postoperative rehabilitation with early oral feeding and early ambulation.
Other modalities
Sham feeding: Postoperative gum chewing or sham feeding is thought to influence GI motility and hormonal activity in a somewhat similar manner to early feeding viz. by stimulating a cephalic-vagal reflex and by inducing the secretion of various GI hormones. Although the results from various studies are conflicting [15-17] a metaanalysis of five published trials suggested a benefit in terms of time to first passage of flatus, first passage of stool and length of hospital stay [15] in spite of methodological differences between studies. Nevertheless, the use of gum seems to be harmless, inexpensive and attractive although larger randomized studies are required before a routine use is recommended.
Acupuncture: Although the potential role of acupuncture in the treatment of paralytic ileus is yet to be defined, it remains a popular and is widely accepted as an effective option in China and parts of Southeast Asia. Published reports are scant but in one report out of 13 patients (n=26) treated with acupuncture, normal bowel function was restored in 12 patients within 72 postoperative hours, compared with 6 out of 13 patients in the control group [15].
Massage: Mechanical massage of the abdominal wall is believed to improve postoperative bowel function and is proved in one report of randomized controlled study involving 50 patients following colectomy. Patients in the study group had a significantly shorter time to first passage of gas [15].
Bran: It was shown in two observational studies that after the use of 8-10 days of preoperative bran supplementation patients are more likely to pass flatus within 24 hours [15, 16].
Visceral learning: When patients were counseled preoperatively that they would have a rapid return of normal GI motility, that they would not have excessive pain and that they would be hungry soon after surgery and be able to eat their favourite dishes, it was shown in a small study that the time to pass first flatus was significantly shorter [15].
A classification of the above modalities and their relative benefits based on published evidence is shown in Table 3.
Table 3: Treatment modalities for postoperative ileus.
|
Beneficial
1. Minimally invasive surgery
2. Epidural analgesia
3. Multimodal therapy
4. Opioid antagonists (only if opiates are used for pain relief)
5. Cisapride (withdrawn because of cardiac side effects)
Probably beneficial
1. Early enteral feeding
2. Early ambulation (may decrease other complications)
3. Neostigmine (use limited because of side effects)
4. Ceruletide (significant nausea and vomiting)
5. Laxatives
6. NSAIDS
7. COX-2 inhibitors
Not beneficial
1. Nasogastric decompression (may increase pulmonary complications)
2. Metoclopramide (only antiemetic)
3. Erythromycin
4. Somatostatin
Unconventional methods (Probably beneficial; need further trials/difficult to prove)
1. Sham feeding (Chewing gum)
2. Visceral learning
3. Electrical stimuli
4. Guided imaging
5. Physical massage
6. Acupuncture
|
Summary of management: There are few data from well-designed studies to guide therapy of patients with prolonged ileus. The goal of initial evaluation is to diagnose secondary treatable causes and rule out mechanical obstruction.
• If a patient after abdominal surgery continues to have ileus after 5 days, a plain abdominal radiograph should be done to delineate the gas pattern. If it suggests ileus or small bowel obstruction, a Gastrografin study may be used to differentiate between the two conditions and possibly to relieve both.
• It is important to consider the nature of the index operation and whether the primary problem was satisfactorily addressed to decide on early reoperation or continue non-operative management. Septic unsettled abdomens and operations known to be more prone for internal herniations than others should have a lower threshold for reoperation (Table 4).
Table 4: Decision for surgery in postoperative ileus
|
Primary operation
|
Question
|
Consideration
|
|
Laparotomy for Small Bowel Obstruction
|
Was the obstructing point dealt with?
|
If not, consider an earlier reoperation unless it was a frozen abdomen.
|
|
Abdominoperineal resection
|
Is the small bowel likely to be prolapsing into a pelvic space (CT)?
|
If yes, consider an earlier reoperation
|
|
Colostomy, ileostomy
|
Is the small bowel caught behind the stoma (contrast/CT)?
|
If yes, consider an earlier operation
|
|
Appendicectomy
|
Is there a pelvic abscess or stump phlegmon?
|
If yes, consider percutaneous drainage and/or antibiotics
|
|
Laparoscopy
|
Is the bowel caught in a trocar site (CT)?
|
If yes, operate immediately
|
|
Radiation enteritis
|
How severe and extensive was the process? Is it ‘resectable’?
|
If no, consider prolonged non-operative management
|
|
Carcinomatosis
|
How severe and extensive was the process? Is it ‘resectable’?
|
If no, continue prolonged palliative–symptomatic approach
|
|
‘Frozen’ abdomen
|
Was the abdomen ‘frozen’ during index operation?
|
If yes, consider prolonged non-operative management
|
|
Intestinal anastomosis
|
Is the anastomosis the cause (contrast/CT)?
|
If yes, consider non-operative management as most resolve spontaneously within 1–2: weeks
|
• A contrast enhanced CT with oral Gastrografin is done
o if clinical sepsis is present
o if strangulating obstruction is likely, based on index operation or
o if the problem has occurred after laparoscopic surgery (to rule out port site incarcerations)
• An NG tube is inserted to decompress the stomach, relieve vomiting, prevent aerophagia and to measure the effluent. Trial clamping and feeding around the tube are not recommended, as they may increase the chances of aspiration.
• Electrolyte imbalances are corrected.
• Use of opiates is restricted and specific antagonists are considered as they are the commonest promoters of ileus.
• Hypoalbuminemia is corrected by TPN which should be started no later than one week postoperatively. One should be wary of hypoalbuminaemic enteropathy that leads to oedematous bowel and anastomotic lines and mimics small bowel obstruction.
• Timing of surgery
o Surgery should be delayed once the strangulating causes are ruled out (Table 4). It should be remembered that the risk of strangulation in the setting of early postoperative small bowel obstruction is small and conservative treatment should be continued. There is no consensus on how long to wait and there is wide variation in reoperation rates- apparently related to case mix, local attitudes and retrospective nature of studies. However, spontaneous resolution beyond ten days of the operation is unlikely.
• Hostile abdomen
o One of the most difficult clinical situations to tackle in abdominal surgery both in terms of a decision for reoperation and what to do when forced to in those patients in whom the index operation showed a hostile peritoneal cavity. This group includes peritoneal carcinomatosis, extensive radiation enteritis and a ‘frozen abdomen’ in which intractable small bowel obstruction is caused by dense, vascular and inseparable adhesions that cement the bowel at multiple points. It is important to recognize the situation very early during the course of primary surgery as well as during reoperation and stop futile dissections before multiple enterotomies and extensive serosal tears leading to massive resections are done. Prolonged parenteral nutrition over a period of a few weeks with complete gastrointestinal rest will allow adhesions to mature with the possibility of a safer and more favourable milieu at a later stage. Tuberculous peritonitis is not an uncommon cause in India and every effort should be made to prove the diagnosis. It is a common observation that even two weeks of antitubercular treatment would relieve critical obstructions to a stage of tolerating liquid and low residue diets.
The above principles are incorporated in the algorithm shown in Figure 2.
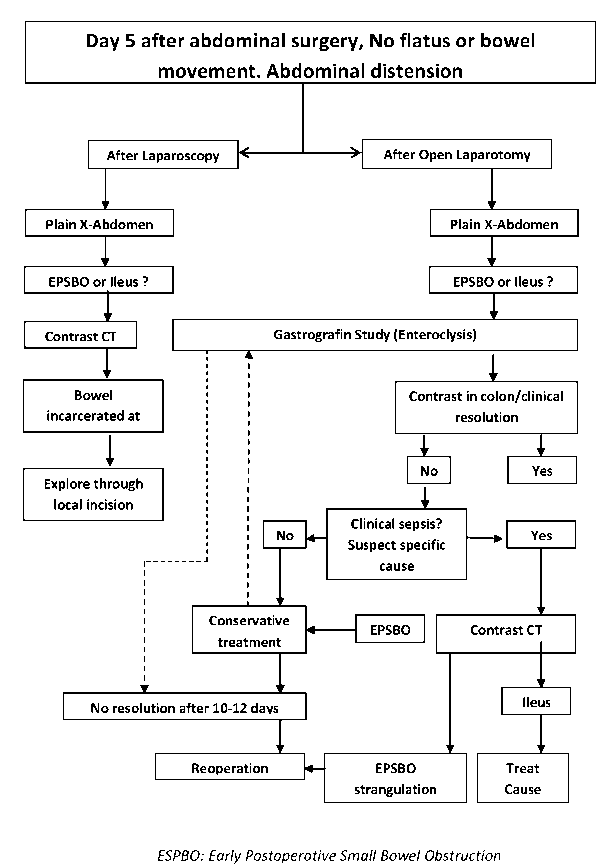
Figure 2: Algorithm for management of postoperative ileus.
Summary
Prolonged postoperative ileus remains a serious clinical problem and is often difficult to differentiate from early postoperative small bowel obstruction. The causes are multifactorial and include combined effects of inhibitory sympathetic inputs, inflammation, opioid analgesics and the type of initial surgery and the surgical techniques employed. In the absence of clear guidelines for treatment when faced with such a problem, an algorithmic approach at diagnosis and management which is predominantly conservative and non-operative yields the best results. Multimodal approach or fast track surgery holds promising results in the prevention of this difficult problem.
Conflicts of interest
Author declares no conflicts of interest.
References
1. Andrews JM, Dent J. Small intestinal motor physiology. In: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Pathophysiology/Diagnosis/Management. Vol II. 7/e. Saunders, Philadelphia 2002.
2. Jørgensen H, Wetterslev J, Møiniche S, Dahl JB. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst Rev 2000, CD001893.
3. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 1998, 228:652.
4. de Jonge WJ, The FO, van der Coelen D, et al. Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice. Gastroenterology 2004, 127:535.
5. Ogilvy AJ, Smith G. The gastrointestinal tract after anaesthesia. Eur J Anaesthesiol Suppl 1995,10:35-42.
6. Condon RE, Cowles V, Ekbom GA, Schulte WJ, Hess G. Effects of halothane, enflurane, and nitrous oxide on colon motility. Surgery 1987, 101:81-85.
7. Delaney C, Kehlet H, Senagore AJ, et al. Postoperative ileus: profiles, risk factors, and definitions—a framework for optimizing surgical outcomes in patients undergoing major abdominal and colorectal surgery. In: Bosker G, editor. Clinical consensus update in general surgery. Roswell (GA): Pharmatecture 2006, 1–26.
8. Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev 2005, CD004929.
9. Ochsner A, Gage IM, Culting RA. Treatment of ileus by splanchnic anesthesia. JAMA 1928, 90:1847-53.
10. Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med 1999, 341:137-341.
11. Althausen PL, Gupta MC, Benson DR, Jones DA. The use of neostigmine to treat postoperative ileus in orthopedic spinal patients. J Spinal Disord 2001, 14:541-545.
12. Furuta Y, Takeda M, Nakayama Y, Ito M, Suzuki Y. Effects of SK-896, a new human motilin analogue ([Leu13]motilin-Hse), on postoperative ileus in dogs after laparotomy. Biol Pharm Bull 2002, 25:1063-1071.
13. Basse L, Thorbøl JE, Løssl K, Kehlet H. Colonic surgery with accelerated rehabilitation or conventional care. Dis Colon Rectum 2004, 47:271.
14. Hansen CT, Sørensen M, Møller C, et al. Effect of laxatives on gastrointestinal functional recovery in fasttrack hysterectomy: a double-blind, placebo - controlled randomized study. Am J Obstet Gynecol 2007, 196:311.e1.