Full Text
Introduction
Diabetic retinopathy (DR), a major microvascular complication, is among the leading causes of vision loss worldwide. With the global prevalence of diabetes escalating, the burden of DR is expected to rise significantly, posing a substantial challenge to public health systems [1]. Diabetic macular edema (DME), characterized by fluid accumulation and thickening of the macula, is the most common cause of vision impairment in individuals with DR [2]. It primarily affects the working population, contributing to reduced quality of life and economic productivity [3].
The pathophysiology of DME involves chronic hyperglycemia-induced retinal ischemia and hypoxia, which trigger the release of vascular endothelial growth factor (VEGF). VEGF disrupts the blood-retinal barrier, induces neovascularization, and increases vascular permeability, culminating in macular edema. As such, targeting VEGF has become the cornerstone of DME management [4].
Anti-VEGF agents have revolutionized the treatment of DME, offering significant improvements in visual acuity and central macular thickness (CMT) [5]. Ranibizumab, a humanized monoclonal antibody fragment, was among the first anti-VEGF therapies approved for intravitreal use. It binds VEGF-A isoforms, preventing them from interacting with VEGF receptors, thereby reducing vascular leakage and neovascularization. Large-scale clinical trials have validated its efficacy and safety in improving visual outcomes in DME patients [6-8]. However, the high cost of ranibizumab and the need for repeated injections impose a financial burden on patients, particularly in developing countries like India.
To address this limitation, biosimilars have emerged as cost-effective alternatives to reference biologics. A biosimilar is defined as a biological product that is highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, efficacy, or immunogenicity [9]. Razumab®, the first ophthalmic biosimilar of ranibizumab, was developed in India and approved by the Drug Controller General of India in 2015. Designed to offer a more affordable option, Razumab® has shown promising results in multiple retinal pathologies, including DME, age-related macular degeneration (AMD), and macular edema secondary to retinal vein occlusions (RVOs). Studies have demonstrated its efficacy in reducing CMT and improving visual acuity, comparable to that of the innovator drug [10, 11].
Rationale of our study
Despite the growing adoption of Razumab®, comparative studies between innovator ranibizumab and Razumab® in treatment-naïve DME patients remain limited. Understanding the real-world effectiveness of these agents is crucial, particularly in resource-constrained settings where cost considerations heavily influence treatment decisions.
This study aims to bridge that gap by providing a prospective comparison of these two agents in a real-world setting. By evaluating key parameters such as visual acuity and central subfield thickness (CSFT) over 12 weeks, our study seeks to determine whether Razumab® offers a cost-effective yet efficacious alternative to ranibizumab in the management of DME, thereby addressing a critical need in ophthalmic care.
Methodology
This prospective observational study was conducted in the Department of Ophthalmology at a tertiary care hospital in India, from March 2023 to September 2024. The study enrolled 60 treatment-naïve diabetic macular edema (DME) patients, who were equally divided into two groups: the Ranibizumab group (n = 30) and the Razumab® group (n = 30). Ethics committee approval was obtained from the institutional ethics committee, and written informed consent was obtained from all participants.
Eligibility was determined based on predefined inclusion and exclusion criteria. Patients aged 18 years or older with central subfield thickness (CSFT) greater than 300 µm and no prior treatment for DME were included. Patients were excluded if they had advanced diabetic eye disease (ADED), co-existing retinal pathologies (e.g., hypertensive retinopathy, retinal vein occlusion [RVO], or neovascular age-related macular degeneration [nAMD]), significant media opacities, active or previous intraocular inflammation, or systemic illnesses such as end-stage renal disease, cerebrovascular accidents, or coronary artery disease within the past six months. Additionally, individuals with a history of anti-VEGF injections or laser therapy were excluded. Although baseline blood sugar levels or HbA1c were not included as specific criteria for inclusion or exclusion.
Comprehensive ophthalmic evaluations were performed at baseline and follow-up visits. These included visual acuity assessment (recorded using a Snellen chart and converted to logMAR units), intraocular pressure (IOP) measurement using Goldmann applanation tonometry, anterior segment examination via slit-lamp biomicroscopy, fundus examination with indirect ophthalmoscopy and fundus photography, and optical coherence tomography (OCT) for CSFT measurement using Heidelberg Spectralis OCT.
Eligible patients were allocated into the Ranibizumab and Razumab® groups using alternate assignment, with every consecutive eligible patient assigned to different groups in an alternating sequence. Each patient received three intravitreal injections of either Ranibizumab or Razumab® (dose: 0.5 mg in 0.05 mL) administered monthly under aseptic conditions. The procedure included topical anaesthesia, ocular surface sterilization, and injection at 3.5–4.0 mm posterior to the limbus, depending on lens status. Follow-up visits were scheduled at weeks 4, 8, and 12 to monitor visual acuity, CSFT, and IOP. Any post-procedural adverse effects were recorded.
Statistical analysis was performed using IBM SPSS version 22. Quantitative data were expressed as means and standard deviations, while categorical variables were presented as frequencies and proportions. Independent t-tests were used to compare changes in visual acuity and CSFT between groups for normally distributed data. A p-value of less than 0.05 was considered statistically significant.
This methodological approach ensured a robust comparison of the efficacy and safety profiles of Ranibizumab and Razumab® in the management of DME.
Results
Demographic and baseline characteristics
A total of 60 patients were enrolled, evenly divided into two groups: Ranibizumab (n = 30) and Razumab® (n = 30). The mean age of participants in the Ranibizumab group was 56.9 ± 7.39 years, while the Razumab® group had a mean age of 59.9 ± 7.82 years, with no statistically significant difference (p = 0.109). Both groups had a similar gender distribution, with 53.3% male and 46.7% female participants (p = 1.000).
The mean duration of diabetes was comparable between groups: 8.6 years in the Ranibizumab group and 8.5 years in the Razumab® group (p = 0.542). Co-morbidities such as hypertension (Ranibizumab: 36.7%; Razumab®: 23.3%; p = 0.260) and dyslipidemia (Ranibizumab: 16.7%; Razumab®: 10.0%; p = 0.448) were similarly distributed across both groups.
The grades of diabetic retinopathy—including moderate non-proliferative diabetic retinopathy (NPDR), severe NPDR, and proliferative diabetic retinopathy (PDR)—were also comparable between the groups (p = 0.955) (Table 1).
Table 1: Comparison of demographic data between two groups.
|
Variable
|
Ranibizumab
|
Razumab®
|
p value
|
|
Age (years)
|
60.5± 5.3
|
62.2± 6.8
|
0.109
|
|
Gender (M,F %)
|
53.3, 46.7
|
55.3, 44.7
|
1.000
|
|
Duration of DM
|
8.5±2.6
|
8.2±1.9
|
0.542
|
|
Hypertension (%)
|
36.7
|
23.3
|
0.260
|
|
Dyslipidemia (%)
|
16.7
|
10
|
0.448
|
|
Grade of retinopathy (%)
|
|
Moderate NPDR
|
30
|
26.7
|
0.955
|
|
Severe NPDR
|
50
|
53.3
|
|
PDR
|
20
|
20
|
Abbreviations: M – Male, F – Female, DM – Diabetes Mellitus, NPDR – Non Proliferative Diabetic Retinopathy, PDR – Proliferative Diabetic Retinopathy.
Visual acuity (logMAR)
Both groups demonstrated significant improvements in best-corrected visual acuity (BCVA) over the 12-week period. In the Ranibizumab group, baseline visual acuity was 0.77 ± 0.10 logMAR, improving to 0.32 ± 0.06 logMAR by week 12. Similarly, the biosimilar group showed an improvement from 0.78 ± 0.10 logMAR at baseline to 0.34 ± 0.06 logMAR at week 12. There were no statistically significant differences in visual acuity outcomes between the two groups at any time point (p > 0.05) (Figure 1).
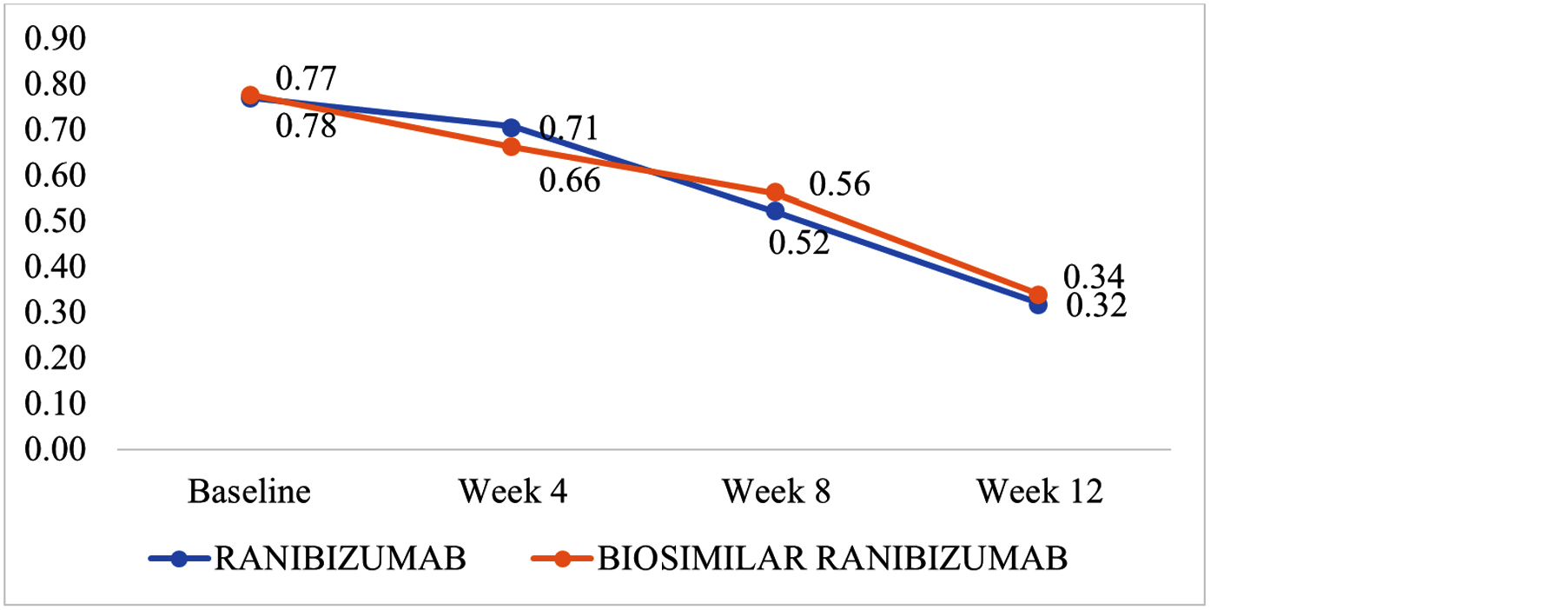
Figure 1: Best corrected visual acuity (logMAR units) between groups at baseline, week 4, week 8 and week 12.
Central subfield thickness
The central subfield thickness (CSFT), measured using spectral-domain optical coherence tomography (SD-OCT), decreased significantly in both groups over the study period. In the Ranibizumab group, CSFT reduced from a baseline of 447.43 ± 61.87 µm to 314.00 ± 24.74 µm at week 12. In the biosimilar group, it decreased from 449.47 ± 47.12 µm at baseline to 30.13 ± 27.17 µm at week 12. Although both reductions were substantial, the difference between the groups was not statistically significant (p > 0.05) (Figure 2).
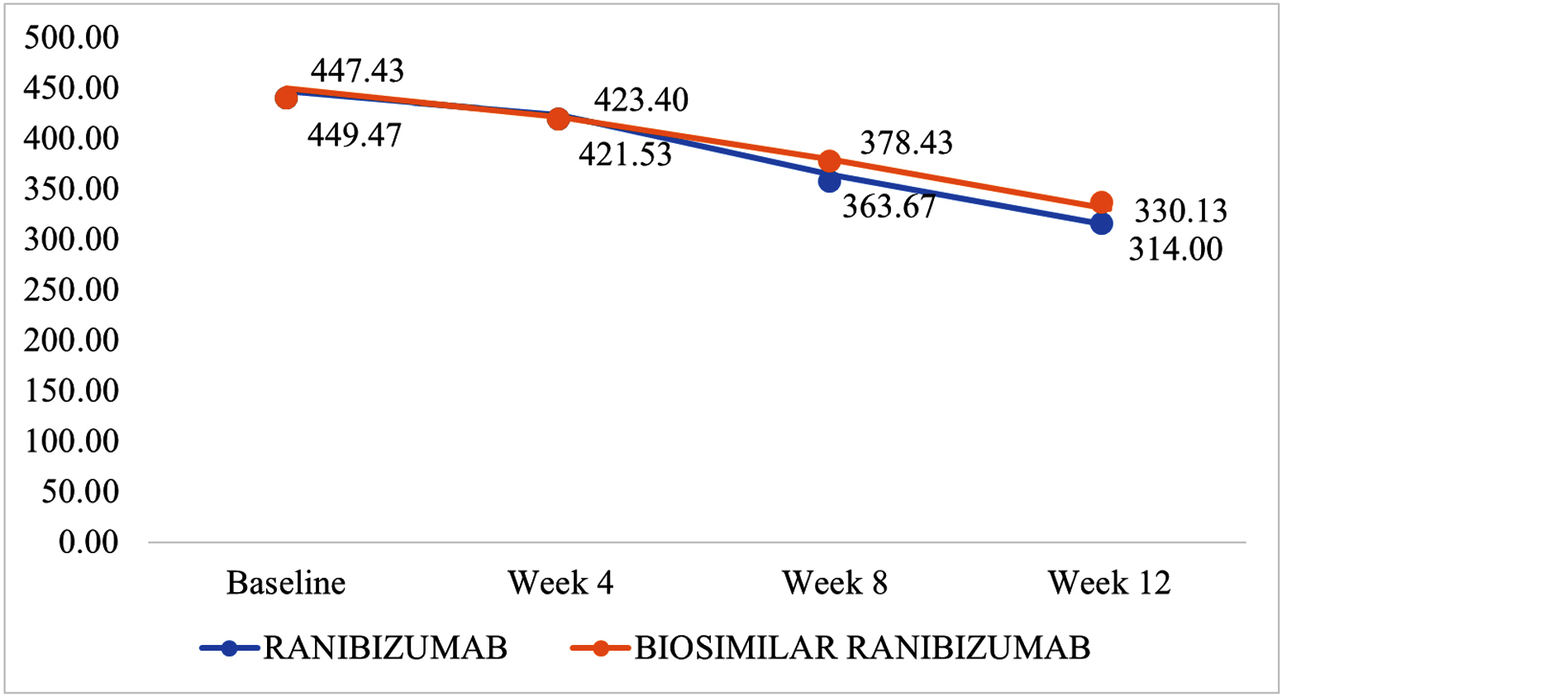
Figure 2: Central subfoveal thickness (microns) between groups at baseline, week 4, week 8, week 12.
Intraocular pressure
Intraocular pressure (IOP) remained stable and within normal limits in both groups throughout the study period. In the Ranibizumab group, IOP was 14.50 ± 2.64 mmHg at baseline and decreased slightly to 13.67 ± 2.48 mmHg by week 12. Similarly, the biosimilar group had an initial IOP of 15.33 ± 2.97 mmHg, which decreased to 12.97 ± 2.09 mmHg at week 12. No statistically significant differences in IOP were observed between the groups at any follow-up point (p > 0.05) (Table 2).
Table 2: Intraocular pressure(IOP) between groups at baseline, week 4, week 8 and week 12.
|
IOP
|
Group
|
P value
|
|
Ranibizumab
|
Razumab®
|
|
Mean (mmHg)
|
Standard deviation
|
Mean (mmHg)
|
Standard deviation
|
|
Baseline
|
14.50
|
2.64
|
15.33
|
2.97
|
0.179
|
|
Week 4
|
14.66
|
2.64
|
14.17
|
2.32
|
0.214
|
|
Week 8
|
15.23
|
2.86
|
14.63
|
2.87
|
0.175
|
|
Week 12
|
13.67
|
2.48
|
12.97
|
2.09
|
0.312
|
Safety: No adverse drug reactions or safety concerns were reported during the study period in either group.
Discussion
Our study provides a detailed comparison of innovator Ranibizumab and Razumab® in the management of treatment-naïve diabetic macular edema (DME), contributing to the growing evidence supporting the clinical utility of biosimilars in ophthalmology. Our findings reveal that both agents demonstrate comparable efficacy and safety profiles, aligning with the results of previous investigations into anti-VEGF therapies for DME.
Efficacy in visual acuity (BCVA): In our study, the mean best-corrected visual acuity (BCVA) in logMAR units improved significantly in both groups over 12 weeks, with no statistically significant differences between them (Ranibizumab: 0.77 ± 0.10 to 0.32 ± 0.06; biosimilar: 0.78 ± 0.10 to 0.34 ± 0.06; p > 0.05). These results are consistent with the outcomes of the RESOLVE trial, where Ranibizumab showed an improvement of +10.3 letters after 12 months compared to sham treatment [12]. Similarly, the RE-ENACT study by Sharma et al. demonstrated a significant improvement in BCVA with Razumab® over 12 weeks (mean baseline: 0.75 ± 0.01; week 12: 0.49 ± 0.01) [13]. Notably, the findings of our study reaffirm the non-inferiority of Razumab® to innovator Ranibizumab in improving visual acuity, as also seen in the study by Sameera et al., where BCVA gains between the two groups were comparable (mean baseline: 0.67 ± 0.41; day 30: 0.57 ± 0.37) [14].
Reduction in central subfield thickness (CSFT): The reduction in CSFT observed in our study was significant in both groups over 12 weeks, with no statistically significant difference between them (Ranibizumab: 447.43 ± 61.87 µm to 314.00 ± 24.74 µm; biosimilar: 449.47 ± 47.12 µm to 330.13 ± 27.17 µm; p > 0.05). This aligns with the RE-ENACT 2 study, which showed significant improvements in CSFT with Razumab® over 48 weeks (mean baseline: 467.09 ± 159.6 µm; week 48: 296.56 ± 49.7 µm) [15]. Moreover, a study by Verma et al. showed similar results, with CSFT reductions from 405.68 µm to 271 µm over three months using Razumab® [16].
Safety profile: No serious ocular or systemic adverse events were reported in either group during the study, confirming the safety of both agents. These findings are comparable to those of the CESAR study by Verma et al., which reported no ocular toxicity or systemic adverse events in patients treated with Razumab® [16]. Additionally, the study by Ratra et al. [17] also highlighted the comparable safety profiles of Razumab® and innovator Ranibizumab in treating retinal diseases.
Comparative analysis: Our findings align closely with the outcomes of other studies comparing Razumab® to innovator Ranibizumab in DME. The retrospective analysis by Chakraborthy et al. confirmed the non-inferiority of Razumab® in terms of BCVA and CSFT outcomes over 12 months [18, 19].
Clinical implications: This study supports the use of Razumab® as a cost-effective and efficacious alternative to innovator Ranibizumab for treating DME. Given the comparable efficacy, safety, and potential cost savings, biosimilars can play a critical role in improving access to treatment, particularly in resource-limited settings.
Limitations: This study has certain limitations, including its relatively small sample size, short follow-up duration, and single-center design, which may limit the generalizability of the findings. These factors highlight the need for further research to validate these results through larger, multicenter, and longer-term studies. Future Directions: Future studies should focus on evaluating the long-term efficacy and safety of Razumab® in diverse patient populations. Additionally, cost-effectiveness analyses would provide valuable insights into the broader integration of biosimilars into routine clinical practice.
Conclusion
This study highlights that Razumab® matches the therapeutic benefits of Ranibizumab for managing diabetic macular edema, demonstrating similar improvements in visual acuity and retinal thickness without compromising safety. Its affordability positions it as a practical option to expand treatment access. Future investigations should explore its long-term outcomes and potential applications in broader clinical settings.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995; 102:7–16.
[2] Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy: IV. diabetic macular edema. Ophthalmology. 1984; 91:1464–1474.
[3] Eisma JH, Dulle JE, Fort PE. Current knowledge on diabetic retinopathy from human donor tissues. World J Diabetes. 2015; 6:312–320.
[4] Wu MY, Yiang GT, Lai TT, Li CJ. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev. 2018; 2018:3420187.
[5] Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372:1193–1203.
[6] Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology. 2009; 116:2175–2181.
[7] Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013; 120:2013–2022.
[8] Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. 2015; 372:1193–1203.
[9] Sharma A, Kumar N, Parachuri N, Bandello F, Kuppermann BD, et al. Biosimilars for retinal diseases: an update. Am J Ophthalmol. 2021; 224:36–42.
[10] Soman M, Nair I, Sheth JU, Nair U. Innovator versus Razumab® in polypoidal choroidal vasculopathy: real-world evidence. Ophthalmol Ther. 2022; 11:591–600.
[11] Gopal S, Kasturirangan S, Madhivanan N, Henry H, Nivean P, et al. Clinical effectiveness and safety of Razumab (a biosimilar of ranibizumab). TNOA J Ophthalmic Sci Res. 2020; 58:154–159.
[12] Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010; 33:2399–2405.
[13] Sharma S, Khan MA, Chaturvedi A. Real-life clinical effectiveness of Razumab® (the world’s first biosimilar of ranibizumab) in retinal vein occlusion: a subgroup analysis of the pooled retrospective RE-ENACT study. Ophthalmologica. 2018; 241:24–31.
[14] Sameera V, Apoorva A, Joshi S, Guruprasad A. Safety and efficacy of Razumab – the new biosimilar in India: our experience. Kerala J Ophthalmol. 2016; 28:180–183.
[15] Sharma S, Khan M, Chaturvedi A, RE-ENACT 2 Study Investigators Group. A multicenter, retrospective study (RE-ENACT 2) on Razumab™ (world’s first Razumab®) in retinal vein occlusion. Ophthalmol Ther. 2020; 9:625–639.
[16] Verma L, Thulasidas M, Purohit A, Gupta A, Narula R, et al. Clinical efficacy and safety of Razumab® (CESAR) study: our experience with the world’s first Razumab®. Indian J Ophthalmol. 2021; 69:347–351.
[17] Ratra D, Roy K, Giridhar S, Madaan S, Bhende P, et al. Comparison between ranibizumab biosimilar, innovator ranibizumab and bevacizumab in a real-world situation. Ophthalmol Ther. 2022; 11:135–149.
[18] Chakraborty D, Sengupta S, Mondal S, Boral S, Das A, et al. Comparison of innovator vs. Razumab® in treating diabetic macular edema: a multicenter retrospective study. Ophthalmol Ther. 2022; 11:629–638.
[19] Chakraborty D, Stewart MW, Sheth JU, Sinha TK, Boral S, et al. Real-world safety outcomes of intravitreal ranibizumab biosimilar (Razumab) therapy for chorioretinal diseases. Ophthalmol Ther. 2021; 10:337–348.