Orginal Research
2024
March
Volume : 12
Issue : 1
Retinal vascular changes in persons with cerebral small vessel disease
Kandela KT, Varghese CP, Chacko F, Mathai MT
Pdf Page Numbers :- 77-81
Krishna Teja Kandela1, Prasanth Varghese C1,*, Fiju Chacko1 and Monsy Thomas Mathai2
1Department of Neurology, Jubilee Mission Medical College & research Institute, Thrissur, Kerala - 680005, India
2Department of Ophthalmology, Jubilee Mission Medical College & Research Institute, Thrissur, Kerala - 680005, India
*Corresponding author: Dr. Prasanth Varghese C, Asst. Professor, Department of Neurology, Jubilee Mission Medical College & Research Institute, Thrissur, Kerala - 680005, India. Email: prasanthneuro@gmail.com
Received 13 September 2023; Revised 6 November 2023; Accepted 13 November 2023; Published 22 November 2023
Citation: Kandela KT, Varghese CP, Chacko F, Mathai MT. Retinal vascular changes in persons with cerebral small vessel disease. J Med Sci Res. 2024; 12(1):77-81. DOI: http://dx.doi.org/10.17727/JMSR.2024/12-14
Copyright: © 2024 Kandela KT et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The retinal and cerebral microvasculature share many morphological and physiological properties, Hence, it is thought that studying these retinal vessels will provide a direct measure to evaluate the vascular and neuronal status of the brain. In the present study, we aimed to study retinal vasculature by fundus examination in persons identified to have cerebral small vessel disease on MRI brain and an attempt to correlate the retinal vascular changes with the degree of MRI brain changes. A case control study was conducted in the department of Neurology, Jubilee Mission Medical College & Research Institute. A total of 180 patients, included 90 patients for case group (Cerebral small vessel disease on MRI brain) and 90 patients for control group (no cerebral small vessel disease on MRI brain) reporting to the neurology department were included in the study. Cerebral small vessel disease was identified and classified/graded as per the modified Fazeka Scale. Ophthalmoscopic examination was done using fundus camera with the help of the ophthalmologist. Results were expressed in percentages and the data obtained was analysed using standard analytical techniques using SPSS software. Majority of cases and controls were in the age group of 61-70 years. Majority of the cases were males 55 (61.1%). Majority of the controls were females 51 (56.7%). 42 (46.7%) cases and 34 (37.8%) controls were hypertensives. Cases with CSVD had a statistically significant association with arteriolar narrowing, AV crossing changes and optic disc edema when compared to controls. Retinal vascular changes were associated with increased cerebral vascular events. These associations persist after accounting for confounding variables known to be disease causing in both circulations.
Keywords: retinal vascular; cerebral small vessel disease; cerebral arterioles
Full Text
Introduction
The term cerebral small vessel disease (CSVD) refers to a syndrome of clinical and imaging findings which are considered to result from pathologies arising from perforating cerebral arterioles, capillaries and venules [1]. CSVD includes white matter lesions (WML) and lacunar infarcts, and is a commonly encountered finding on computed tomography (CT) and magnetic resonance imaging (MRI) scans especially in case of elderly patients [2]. The disease is also found to be associated with vascular risk factors like systemic hypertension, diabetes mellitus, atherosclerosis of vessels and atrial fibrillation [3, 4]. In CSVD the symptoms are mainly due to infarction of subcortical structures. There is increasing evidence that the clinical and pathogenetic pattern, and risk factor profile, of stroke varies among different ethnic groups [5]. Asians, for example, have a high prevalence of intracranial large artery disease and cerebral small vessel disease and a stronger relationship between blood pressure and stroke risk [6, 7]. The retinal blood vessels, measuring 100 to 300 µm in size, offer a unique and easily accessible window to study correlates and consequences of cerebral microvascular disease, because the retina and brain share similar anatomic features and physiological properties [8]. In the last decade, there have been several population-based and clinical studies in white populations which have consistently shown that retinal microvascular signs (eg., retinopathy and retinal venular widening), assessed from retinal photographs, are predictors of clinical stroke events, stroke death and are associated with subclinical measures of cerebral small vessel disease [9]. In a study by Magdalena et al retinal nerve fibre layer thickness assesses by OCT was correlated with the extent of cerebral small vessel disease [10].
In the present study, we aimed to study retinal vasculature by fundus examination in persons identified to have cerebral small vessel disease on MRI brain and an attempt to correlate the retinal vascular changes with the degree of MRI brain changes.
Materials and methods
This case control study was conducted at the department of Neurology, Jubilee Mission Medical College & Research Institute, Thrissur, following approval from institutional ethical committee, The study was conducted for a period of 18 months from July 2020 to January 2022.
One hundred and eighty patients reporting to the neurology department were included in the study. All patients who have evidence of cerebral small vessel disease on MRI brain were taken as cases (90 cases). Age and gender matched controls with no evidence of cerebral small vessel disease on MRI were taken as controls (90 controls). Cerebral small vessel disease was identified and classified as per the modified Fazeka Scale [11]. Ophthalmoscopic examination was done using fundus camera with the help of ophthalmologist. Retinal vascular findings were correlated with cerebral small vessel disease.
Statistical analysis
Collected data was coded and entered in MS excel. Results were expressed in percentages and the data obtained were analysed using standard analytical techniques using SPSS software at the end of the study. Mean and proportions was calculated for continuous and categorial variables respectively. Difference in means was tested for statistically significant using Chi square test. A p value of < 0.05 was considered to be statistically significant.
Results
It can be observed that there was almost equal distribution of cases and controls with respect to their age. Majority of cases and controls were in the age group of 61-70 years (Figure 1).
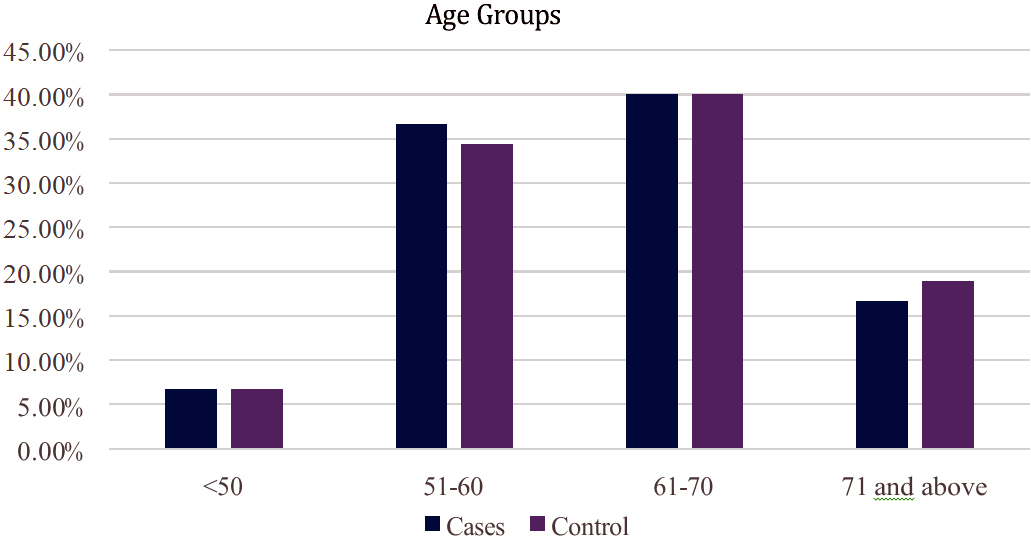
Figure 1: Distribution of cases and controls with respect to their age.
Comparison of arteriolar narrowing between cases and controls
From the table 1 it can be observed that, 57 (63.3%) cases and 37 (41.1%) controls were found to have arteriolar narrowing. This association was found to be statistically significant.
Table 1: Arteriolar narrowing among cases and controls.
|
Arteriolar narrowing
|
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
33 (36.7)
|
53 (58.9)
|
86 (47.8)
|
8.90
|
0.001
|
|
Yes
|
57 (63.3)
|
37 (41.1)
|
94 (52.2)
|
|
Total
|
90
|
90
|
180
|
Comparison of AV crossing changes between cases and controls
From the table 2 it can be observed that, 43 (47.8%) cases and 17 (18.9%) controls were found to have AV crossing changes. This association was found to be statistically significant.
Table 2: AV crossing changes between the groups.
|
AV crossing
changes
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
47 (52.2)
|
73 (81.1)
|
120 (66.7)
|
16.90
|
0.001
|
|
Yes
|
43 (47.8)
|
17 (18.9)
|
60 (33.3)
|
|
Total
|
90
|
90
|
180
|
Comparison of Vessel tortuosity between cases and controls
12 (13.3%) cases and 7 (7.8%) controls were found to have vessel tortuosity (Table 3) and this association was not statistically significant.
Table 3: Vessel tortuosity data.
|
Vessel tortuosity
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
78 (86.7)
|
83 (92.2)
|
161 (89.4)
|
1.47
|
0.225
|
|
Yes
|
12 (13.3)
|
7 (7.8)
|
19 (10.6)
|
|
Total
|
90
|
90
|
180
|
Comparison of sheathing of vessels
From the table 4 it can be observed that, none of the cases and 4(4.4) controls were found to have sheathing of arteriole/ venule and this association was not significant.
Table 4: Sheathing of vessels among the groups.
|
Sheathing of
arteriole/ Venule
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
90
|
86 (95.6)
|
176 (97.8)
|
5.63
|
0.060
|
|
Yes
|
0
|
4 (4.4)
|
4 (2.2)
|
|
Total
|
90
|
90
|
180
|
Comparison of occlusion of vessels
From the table 5 it can be observed that, 6 (6.7%) cases and 4 (4.4%) controls were found to have occlusion of arteriole/venule. P value for the association is 0.515 and the association is not significant.
Table 5: Data on occlusion of arterioles and venules.
|
Occlusion of
arteriole/ Venule
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
84 (93.3)
|
86 (95.6)
|
170 (94.4)
|
0.424
|
0.515
|
|
Yes
|
6 (6.7)
|
4 (4.4)
|
10 (5.6)
|
|
Total
|
90
|
90
|
180
|
Comparison of microaneurysm between cases and controls
From the table 6 it can be seen that, 2 (2.2%) cases and 13 (14.4%) controls were found to have microaneurysm. This association was found to be statistically significant.
Table 6: Comparison of microaneurysms between the groups.
|
Microaneurysm
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
88 (97.8)
|
77 (86.6)
|
165 (91.7)
|
8.800
|
0.003
|
|
Yes
|
2 (2.2)
|
13 (14.4)
|
15 (8.3)
|
|
Total
|
90
|
90
|
180
|
Comparison of optic disc edema between cases and controls
From the table 7 it can be concluded that, 12 (13.3%) cases and 3 (3.3%) controls were found to have optic disc edema. This association was found to be statistically significant.
Table 7: Optic disc edema between cases and controls.
|
Optic disc
edema
|
Group
|
Total
|
Chi square
|
p value
|
|
Cases
|
Control
|
|
No
|
78 (86.7)
|
87 (96.7)
|
165 (91.7)
|
5.819
|
0.015
|
|
Yes
|
12 (13.3)
|
3 (3.3)
|
15 (8.3)
|
|
Total
|
90
|
90
|
180
|
Discussion
Presently, the pathogenesis of cerebral small vessel disease (CSVD) in humans is not completely understood, in part due to the difficulty in imaging in vivo the cerebral microvasculature. There is strong evidence that retinal vascular features are associated with stroke, CSVD and stroke subtype [12-15]. Retinal vascular features are also associated with presence and progression of features of SVD [16, 17].
This case control study was carried out with an aim to study the retinal vasculature by fundus examination in persons identified to have cerebral small vessel disease on MRI brain and attempt has been made to correlate the retinal vascular changes with cerebral small vessel disease.
Retinal arteriolar narrowing is a recognized consequence of chronic hypertension and independently predicts cardiovascular morbidity and mortality. Recent studies in adult populations have shown that retinal arteriolar narrowing is strongly associated with past, current, and future blood pressure levels [18-22] and independently predicts incident stroke, coronary heart disease, and cardiovascular mortality [22].
In the present study we found that 57 (63.3%) cases and 37 (41.1%) controls were found to have arteriolar narrowing. In this study 43 (47.8%) cases and 17 (18.9%) controls were found to have AV crossing changes. In the study of Kwa et al, 154 (86%) patients, were found a retinal arterial abnormality, most frequently narrowing (81%), sclerosis (45%), and crossings (44%). Buckling stimulates wall remodeling and the interaction between artery dynamics, buckling and wall remodeling leads to further development of vessel tortuosity [23].
Vessel tortuosity may be caused by multiple factors: genetic factors, degenerative vascular diseases and an alteration in blood flow and pressure. Cerebral CSVD is characterized by thickening of the walls and elongation and tortuosity of the small perforating arteries in the brain, resulting in lacunar infarcts and cerebral WML [24-26]. In the present study, 12 (13.3%) cases and 7 (7.8%) controls were found to have vessel tortuosity.
In a cross sectional study of 179 patients with known cerebrovascular, cardiovascular or peripheral arterial disease, 108 (60%) had cerebral small vessel disease as assessed by white matter changes on MRI and 154 (86%) had retinal artery abnormalities with one or more of focal arteriolar narrowing, altered AV crossings, arteriolar sclerosis, or vessel tortuosity on retinal photographs as evaluated by expert clinicians [27]. Retinal vessel abnormalities were more frequent in those with MRI changes (92%) than those without (77%), Similar observations were made in a substudy of the atherosclerosis risk in communities (ARIC) cohort study [28]. CSVD in diabetic retinopathy is also studied and found that patients with diabetic retinopathy are more likely to have changes of small vessel disease in brain imaging [29].
Limitations of the study: The study was conducted in a tertiary referral centre which may not reflect the general population. This is a single centre study with limited number of patients, multicentric study with a greater number of patients would have given more robust data.
Conclusion
The study found significant retinal vascular changes in CSVD. Differences between participants with CSVD and healthy controls were pronounced. Cases with CSVD had a statistically significant association with arteriolar narrowing, AV crossing changes and optic disc edema when compared to controls. These associations persist after accounting for confounding variables known to be disease causing in both circulations. It is not known whether the relationships identified reflect other confounding variables such as an individual’s susceptibility to vascular disease. Future research is needed to deepen our understanding of these associations on a pathophysiological level and to identify interventions beyond traditional vascular risk factor modification, that, when prompted by identification of retinal vascular disease, may reduce cerebral vascular morbidity and mortality.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Mustapha M, Nassir CM, Aminuddin N, Safri AA, Ghazali MM. Cerebral small vessel disease (CSVD)–lessons from the animal models. Frontiers in physiology. 2019:1317.
[2] Caunca MR, Leon-Benedetti D, Latour L, Leigh R, Wright CB. Neuroimaging of cerebral small vessel disease and age-related cognitive changes. Frontiers in aging neuroscience. 2019:145.
[3] Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018; 34:575–584.
[4] Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Frontiers in physiology. 2019;10:135.
[5] Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circul Res. 2017; 120:472–495.
[6] Kim JS, Caplan LR, Wong KS, editors. Intracranial atherosclerosis: pathophysiology, diagnosis and treatment. Karger Medical and Scientific Publishers; 2016.
[7] Man BL, Fu YP. Concurrent stenoses: a common etiology of stroke in Asians. World J Clin Case. 2014; 2:201.
[8] Cheung CW, Tay WT, Ikram MK, Ong YT, Silva DAD, et al. Retinal microvascular changes and risk of stroke. Stroke. 2013; 44:2402–2408.
[9] Kawasaki R, Cheung N, Mosley T, Islam AF, Sharrett AR, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010; 41:1826–1828.
[10] Langner SM, Terheyden JH, Geerling CF, Kindler C, Keil VCW, et al. Structural retinal changes in cerebral small vessel disease. Sci Rep. 2022; 12:9315.
[11] Kincaid MC, Yanoff M, Fine BS. Retina vascular disease. In: Tasman W, Jaeger EA, eds. Duane’s ophthalmology. CD-ROM ed. Philadelphia: Lippincott–Raven. 1997.
[12] Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psych. 2009; 80:158–165.
[13] Doubal FN, Dhillon B, Dennis MS, Wardlaw JM. Retinopathy in ischemic stroke subtypes. Stroke. 2009; 40:389–393.
[14] Doubal FN, MacGillivray TJ, Patton N, Dhillon B, Dennis MS, et al. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology. 2010; 74:1102–1107.
[15] Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, et al. Retinal fractals and acute lacunar stroke. Annals of neurology. 2010; 68:107–111.
[16] Mutlu U, Cremers LG, De Groot M, Hofman A, Niessen WJ, et al. Retinal microvasculature and white matter microstructure: the Rotterdam Study. Neurology. 2016; 87:1003–1010.
[17] Qiu C, Ding J, Sigurdsson S, Fisher DE, Zhang Q, et al. Differential associations between retinal signs and CMBs by location: The AGES-Reykjavik study. Neurology. 2018; 90:e142–e148.
[18] Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999; 150:263–270.
[19] Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Bri J Ophthalmol. 2002; 86:1007–1013.
[20] Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, et al. atherosclerosis risk in communities study. Retinal arteriolar diameter and risk for hypertension. Annal Int Med. 2004;140:248–255.
[21] Smith W, Wang JJ, Wong TY, Rochtchina E, Klein R, et al. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the blue mountains eye study. Hypertension. 2004; 44:442–447.
[22] Wong TY, Mitchell P. Hypertensive retinopathy. New Eng J Med. 2004; 351:2310–2317.
[23] Kwa VI, Sande JJV, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002; 59:1536–1540.
[24] Ostrow PT, Miller LL. Pathology of small artery disease. Advanc Neurol. 1993; 62:93–123.
[25] Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659.
[26] Warlow CP, Dennis MS, Gijn JV, Hankey GJ, Sandercock PA, et al. Stroke: A practical guide to management. BMJ Int. 1997; 314:1840.
[27] Kwa VIH, Franke CL, Verbeeten B Jr, Stam J, for the Amsterdam Vascular Medicine Group. Silent intracerebral microhemorrhages in patients with ischemic stroke. Ann Neurol. 1998; 44:372–377.
[28] Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002; 288:67–74.
[29] Zhang Y, Zhang Z, Zhang M, Cao Y, Yun W. Correlation between retinal microvascular abnormalities and total magnetic resonance imaging burden of cerebral small vessel disease in patients with type 2 diabetes . Front. Neurosci. 2021; 15:727998.