Orginal Research
2025
March
Volume : 13
Issue : 1
Serum BNP and troponin-I as predictors of outcome in acute exacerbation of chronic obstructive pulmonary disease
Yadav DP, Singh M, Singh A, Prakash V, Srivastava GN
Pdf Page Numbers :- 83-87
Devendra Paratap Yadav1, Mrityunjaya Singh2,*, Anjana Singh3, Ved Prakash2 and Govind Narayan Srivastava4
1Department of Pulmonary Medicine, MPMMM Cancer Centre, Tata Medical Centre, Varanasi, UP - 221005
2Department of Pulmonary & Critical Care Medicine, King George’s Medical University, Lucknow, Uttar Pradesh 226003, India
3Department of Biochemistry, Era’s Medical University, Lucknow, Uttar Pradesh 226003, India
4Department of Respiratory Medicine, Institute of Medical Sciences, Varanasi, Uttar Pradesh 221005, India
*Corresponding author: Dr. Mrityunjaya Singh, MD., Department of Pulmonary & Critical Care Medicine, King George’s Medical University, Lucknow, Uttar Pradesh 226003, India. Email: dr.mrityunjaya@gmail.com
Received 14 October 2024; Revised 22 November 2024; Accepted 2 December 2024; Published 12 December 2024
Citation: Yadav DP, Singh M, Singh A, Prakash V, Srivastava GN. Serum BNP and troponin-I as predictors of outcome in acute exacerbation of chronic obstructive pulmonary disease. J Med Sci Res. 2025; 13(1):83-87. DOI: http://dx.doi.org/10.17727/JMSR.2024/13-14
Copyright: © 2025 Yadav DP et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) poses a major burden due to unpredictable outcomes, prolonged hospitalizations, and mortality risks. Identifying reliable prognostic markers is essential for better management. This study evaluates serum biomarkers—CRP, BNP, fibrinogen, and troponin I—as predictors of AECOPD outcomes to enhance clinical decision-making.
Methods: This was a single-centre, prospective, observational study involving 154 patients admitted with AECOPD to a tertiary care hospital between August 2022 and May 2024. Patients with pre-existing ischemic heart disease, recent myocardial infarction, severe renal or hepatic disease, or neuromuscular disorders were excluded. Biomarkers were measured at the time of admission, and patients were followed up to 90 days post-discharge. The primary endpoint was in-hospital mortality, while secondary endpoints included the need for intensive care and 90-day survival.
Results: Of the 154 patients enrolled, 18% (n=28) succumbed to their illness during hospitalization. The non-survivor group demonstrated significantly higher levels of BNP (mean: 1218.10 ± 559.35 pg/mL; p=0.018) and troponin I (mean: 0.45 ± 0.14 ng/mL; p<0.001) at admission compared to survivors. CRP levels were elevated in both groups but did not independently predict mortality (p=0.08). Fibrinogen levels were higher in non-survivors but were not statistically significant (p=0.187). Notably, BNP exhibited a strong positive correlation with prolonged hospital stay and intensive care requirements. Levels of BNP and fibrinogen normalized at 90-day follow-up in survivors, underscoring their role as acute-phase biomarkers. Troponin I remained elevated in non-survivors, highlighting its potential as a mortality predictor.
Conclusions: Serum BNP and troponin I predict in-hospital mortality and prolonged hospitalization in AECOPD, while CRP and fibrinogen have limited prognostic value. BNP correlates with disease severity, aiding risk stratification and intensive care decisions. Larger multi-center studies are needed to validate findings and develop comprehensive prognostic models for AECOPD management.
Keywords: AECOPD; biomarkers; BNP; troponin I; CRP; fibrinogen; mortality; hospital outcomes
Full Text
Introduction
Chronic obstructive pulmonary disease (COPD) is a major respiratory condition affecting a significant proportion of adult population globally [1]. Although previously considered a major burden in developed countries due to its association with smoking and early diagnosis and reporting, the last few decades have seen developing countries catching up in the race, thanks to growing air pollution and improvement in health infrastructure [2]. Since the inception of GOLD, our understanding of COPD has evolved significantly from just an obstructive airway disease to a systemic inflammatory condition affecting various systems of the body with lung as the primary target [3]. Acute exacerbations of COPD (AECOPD) are episodes in the natural course of the disease characterized by sudden worsening of symptoms beyond day-to-day variation that may warrant change in medication or necessitate hospitalization [4]. An acute exacerbation is a serious event not only because of the immediate life-threatening challenge it poses but also because it triggers a vicious cycle of lowering the baseline airflow limitation which further increases the risk of future exacerbations [5]. Post recovery and discharge, patients may take several weeks to months to get back to near to pre-exacerbation levels of health status [6]. Fiscally, apart from the cost of treatment of exacerbation per-se, post hospitalization costs of maintenance treatment, health support and assisted living add to the monetary burden [7]. Although most common culprit triggering an exacerbation is a respiratory infection, risk factors which make a COPD patient more prone to flare-ups are increased age, severity of airflow limitation (quantified as Forced Expiratory Volume in 1st second; FEV1), persistent symptoms, frequent past exacerbations and comorbidities (mainly cardio-vascular) [8] among many others.
A severe exacerbation of COPD requiring hospitalization is a frustrating task for treating physician as well mostly because despite best management strategies the outcome is unpredictable especially in the first 24 to 48 hours. Patient may require prolonged hospitalization, ICU care and mechanical ventilation. Prognostic indices have been thoroughly studied in cases of stable COPD and too predictor tools for mortality risk, such as the BODE Score, are well established [9]. However, there have been limited research for prognostic markers in cases of exacerbations requiring hospitalization. Clinical assessment and physiological parameters like peak expiratory flow rate (PEFR) and FEV1 are prone to subjective variations [10] and may not be reproducible in severe exacerbations. Biochemical markers drawn from blood can overcome this limitation [11]. Moreover, level of biomarkers in early phase of exacerbations may differ from that in after initial few days.
Therefore, studies to assess prognostic indices in shorter duration of COPD exacerbation are needed in this subset of population. Prognostication of patients admitted with COPD exacerbations will not only help to adequately focus and optimize the use of resources but also to manage expectations of patients and family. The present study was designed keeping these factors in mind, to prospectively study the clinical presentation, arterial blood gas (ABG) analysis, and biomarkers- Fibrinogen, Brain Natriuretic Peptide (BNP), Troponin-I and High sensitivity C-Reactive Protein (hs-CRP) as predictors of outcome in the patients with acute exacerbation of COPD. This study explores the role of serum biomarkers—C-reactive protein (CRP), brain natriuretic peptide (BNP), fibrinogen, and troponin I—as predictors of outcomes in AECOPD.
Materials and methods
This was a single centre prospective observational cross-sectional study conducted at Sir Sunderlal Hospital & Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. The study was approved by Institutional ethics committee and patients were enrolled from August 2022 to May 2024 after obtaining written informed consent. The sample size was calculated by 19.4 % prevalence of COPD. The confidence interval was to be 95% and permissible error was 5%. under these circumstances minimal sample size came out to be 36.
198 Patients above 45 years of age who presented to outpatient department or emergency of our hospital with AECOPD were admitted to general ward, High Dependency unit (HDU) or Respiratory Intensive care Unit (RICU) as per triage. All patients with pre-diagnosed COPD according to GOLD guideline with at least one spirometry report in last 1 year were enrolled. The latest spirometry report in stable condition before exacerbation was used as pre-exacerbation baseline lung function.
Patients with Ischemic heart disease, left ventricular dysfunction, regional wall motion abnormality on 2D-echocardiography, myocardial infarction in last 3 years, sepsis or septic shock at the time of admission, pre-existing renal, hepatic or neuro-muscular disease were excluded from the study.
After exclusion, effective study size (n) was 154. During hospital admission all the patients were managed as per standard protocol including oxygen support, oral or intravenous antibiotics and oral or intravenous steroid, nebulization with bronchodilators and inhaled steroids and supportive measures. Non-invasive and Invasive mechanical ventilation was provided as and when indicated. Primary endpoint was death or discharge. Secondary endpoint was 90 days follow-up.
All data from admission to primary endpoint was recorded. This included Clinical examination, CAT score, routine biochemistry, ABG, Electrocardiogram, Chest X-ray, Echocardiography and special biomarkers (at time of admission)- Fibrinogen, BNP, Toponin-I, hs-CRP.
Patients were classified into two groups Survived or Non-survived depending on in hospital mortality. Survived patient were assessed for need for domiciliary supportive care like Long term oxygen therapy and Non Invasive Ventilation and were followed at 2 weeks, 6 weeks and 90 days after discharge.
Spirometry, biomarkers and CAT score were again obtained at secondary endpoint.
Statistical analysis
Different variables were compared using Pearson’s Correlation. P value < .05 was considered statistically significant. The tests were performed using IBM SPSS software version 16.
Results
General characteristics of the study population are shown in table 1 and special biochemical profile in table 2. A total of 154 admitted patients were enrolled. Out of these 28 (18%) patients died. Mean age of patients in survived group was 62.3±9.3 years with and in non-survival group mean age of patients was 65.1±7.5 years. Age difference in both groups was not significant t=0.836 (p-value of 0.407).
Table 1: Group characteristics.
|
Total (n)
|
154
|
|
Death
|
Survived
|
|
No. of patients
|
28 (18%)
|
126 (82%)
|
|
Mean age
|
64.11 ±7.5
|
57.3±9.35
|
|
Male
|
13
|
65
|
|
Females
|
15
|
61
|
|
Mean FEV1
|
42.24±16.16
|
53.78±9.23
|
|
Mean FVC
|
54.22±12.37
|
59.37±14.16
|
|
Active smoker
|
12
|
19
|
|
TDI
|
16.44±6.71
|
9.24±4.25
|
|
mean No. of exacerbations
|
4.44±2.68
|
2.24±1.2
|
|
CAT score
|
29.77±1.56
|
23.26±2.13
|
|
RVSP
|
35.66± 6.91
|
26.53± 3.88
|
|
Pneumonia/ Consolidation
|
11
|
32
|
|
Emphysematous
|
10
|
56
|
|
Non-emphysematous
|
18
|
70
|
Table 2: Biomarkers.
|
Parameter
|
Death
|
Survived
|
|
At admission
|
90 days
|
|
paO2
|
52.77±8.38
|
66.48±9.09
|
-
|
|
paCO2
|
82.65±16.90
|
56.79±9.45
|
-
|
|
pH
|
7.16±0.92
|
7.32±0.045
|
-
|
|
BNP (pg/mL)
|
1218.10±559.35
|
418.32±137.08
|
281.07±108.2
|
|
Fibrinogen (mg/dL)
|
430.44±99.42
|
365.49±137.08
|
264.58±52.22
|
|
Trop-I (ng/mL)
|
0.45±0.14
|
0.028±0.045
|
0.016±0.022
|
|
hs-CRP (mg/L)
|
2.34±0.74
|
1.43±0.42
|
1.21±0.26
|
The mean total duration of illness (TDI) was higher in non-survived group than survived group and this difference was statistically significant (t=3.697 p=0.001).
The patients in non-survival group had suffered a greater number of exacerbations since 1st diagnosis when compared with survived group 3.55±0.78 vs 1.4±0.61 (Z=-2.317 p=0.03) which was significant.
CAT score at the time of admission was significantly more than that in non-survival group in comparison to survived group 29.26±1.56 vs 27.26±2.13 (t=3.326, p=0.002). At time of discharge CAT score showed significant improvement (mean= 17.65±3.17, t= 18.269, p=0.001). Additionally, CAT Score per memory recall for period before exacerbation, in survival group was (mean) 8.2±3.18 and that at 90 days follow-up in the same patients was (mean) 12.54±1.28.
At the time of admission, PaO2 (t=-3.238, p=0.002) and pH (t= -8.134, p=0.001) levels were significantly lower and PaCO2 levels were significantly higher (t=3.275, p=0.002) in the non-survival group than the survival group.
RVSP values were more increased significantly in non-survival group when compared with survived group (t=2.475, p=0.017).
CRP level was more raised in non-survival group in comparison to survived group and it was not significant (t=6.076, p=0.08). BNP (Z=2.441, p=0.018) and Troponin-I (Z= 4.155, p=0.0001) levels were raised significantly more in non-survival group when compared with survived group, at the time of admission, while Fibrinogen (t==1.339, p=0.187) levels although higher than survival group, were not statistically significant. Out of the 4 biomarkers, BNP levels dropped significantly lower at 90 days follow-up, while CRP, Fibrinogen and Troponin-I although lowered but the decrease was not statistically significant.
The mean duration of hospital stay was 6.51±2.95 days. It showed a weak positive correlation with Fibrinogen level (Figure 1) and a strong positive correlation with BNP levels (Figure 2). Troponin-I and CRP levels did not correlate with duration of hospitalization.
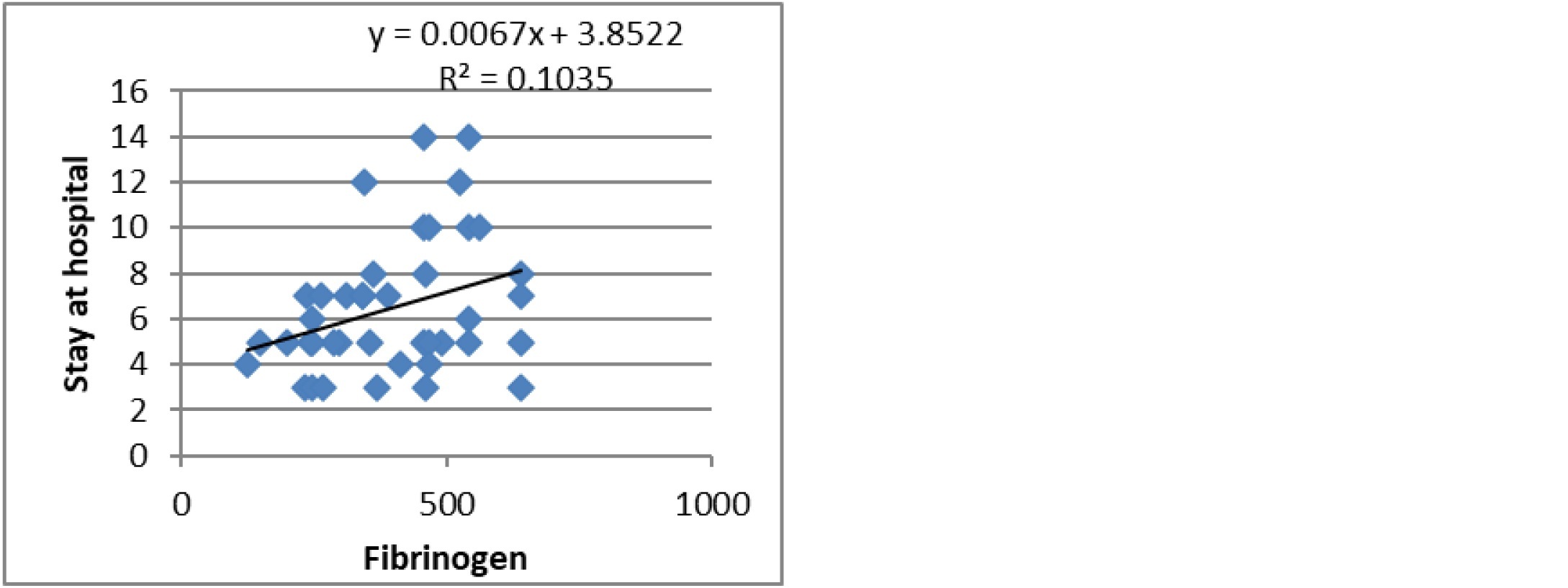
Figure 1: Fibrinogen Vs Hospital stay.
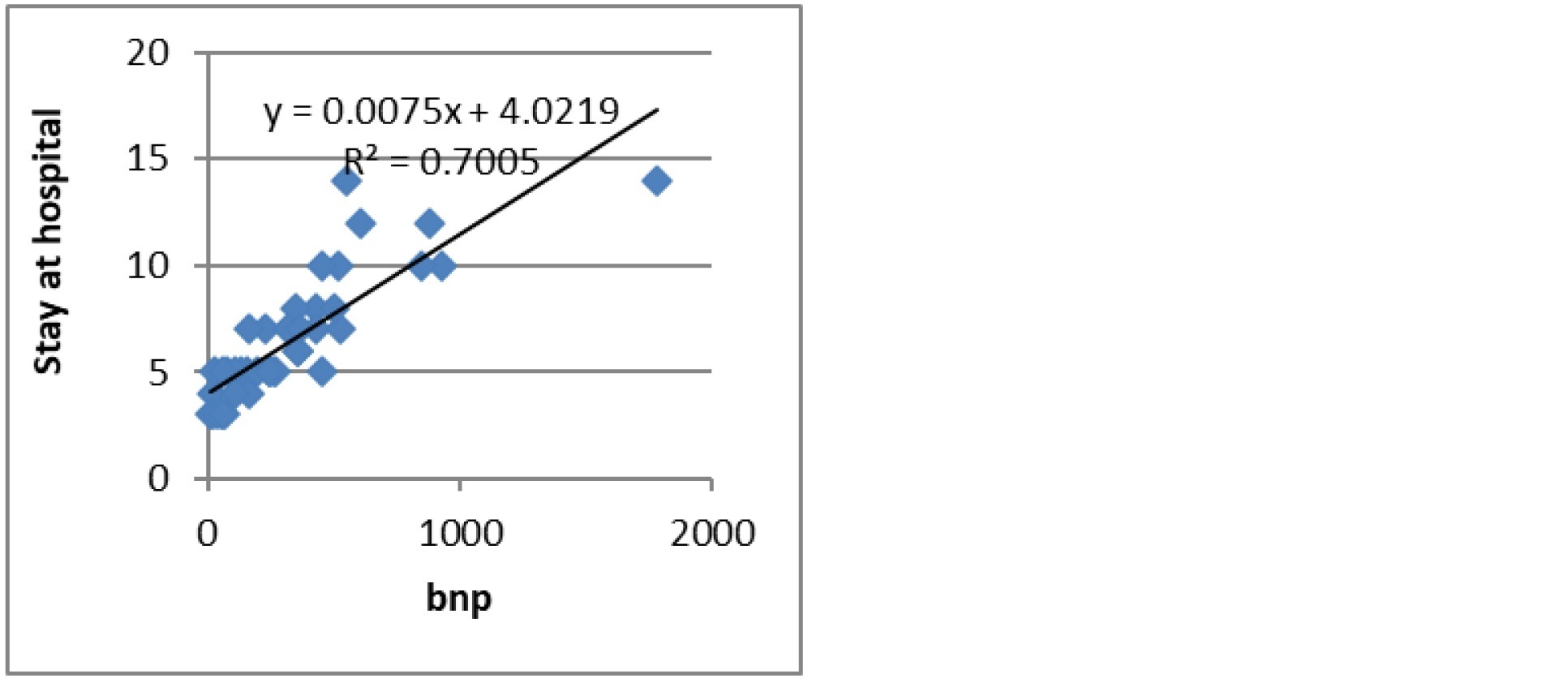
Figure 2: BNP Vs Hospital stay.
Discussion
Developing an easy-to-use tool to identify predictors of outcome after an exacerbation of chronic obstructive pulmonary disease could help clinicians choose specific measures of medical care to decrease mortality and hospital stay in these patients. Our study aimed at identifying these clinical tools along with biochemical markers that could help develop a comprehensive approach regarding best management options and swift distribution of resources in a resource limited setting.
C- reactive protein is a marker of inflammation and correlated with degree of pulmonary inflammation in COPD [12]. Recent studies have shown that CRP is a strong long-term predictor of COPD outcome [13]. In our study CRP was significantly raised in non-survived group, however this could be due to overall inflammation process. Stolz D et al, 2007 did not find CRP to be a predictor of mortality over 6 months period [14]. However, CRP levels were raised in exacerbation. Helmy TA et al [13], showed that CRP levels are significant predictor of mortality in COPD patients admitted in ICU. Interleukin-6 is a multifunctional cytokine that plays a central role in host defence due to its wide range of immune and haemotopoietic activities and ability to induce the acute phase response. Similar results were obtained by Shafiek H and colleagues in 2016 [15].
BNP is a peptide hormone that is produced from the ventricles of heart in response to pressure and volume stress on the cardiomyocytes. As a result, both congestive heart failure (left ventricular failure)and right ventricular failure or hyperthrophy due to pulmonary hypertension secondary to lung diseases can lead to elevations in plasma BNP. In our study BNP was raised in non-survival groups in comparison to survived groups and it was statistically significant. The levels lowered to equivocal and normal range at 90 days follow-up unlike hs-CRP. This demonstrates its utility as an acute phase marker. The death group had mean levels more than 3 times that of survival group at time of admission. In one study Chang CL et al showed that BNP independently predicted short term outcomes including intensive care unit admission, inpatient and 30-day mortality [16]. Another study showed that Median BNP was also significantly higher in failed hospital discharges (either in-patient death or early re-hospitalisation) compared to successful discharges following AECOPD hospitalization [17]. In our study Patient with high BNP showed strong positive correlation with duration of hospital stay. It was also increased in patients who needed Intensive care support [18].
Fibrinogen is acute phase protein. It is increased in response to IL-6 production. In our study Fibrinogen levels were more increased in non-survived group when compared with survived group although it was statistically not significant. Unlike CRP and similar to BNP, the levels at 90 days follow-up returned to normal range which demonstrates its utility as acute phase biomarker but no significant role in predicting mortality or hospital stay. Mannino et al, 2012 in 8504 individuals from NHANES study showed association between fibrinogen level and mortality [19]. In another study Saldias et al, 2011 showed fibrinogen level was higher during exacerbation and it decreased after 30 days[20]. Although we were unable to show this in our study due to lack of post discharge follow up. A recent study (ECLIPSE cohort) demonstrated that while plasma fibrinogen was associated with exacerbations, end product of fibrinogen is predictive of mortality and more likely associated with emphysema phenotype of COPD [21].
Troponin I is marker of cardiac muscle stretch. In our study it was significantly increased in non survived group in comparison to survived group. Baillard et al., in their study cohort of 71 patients with COPD acute exacerbations, found that troponin I values were significantly elevated and were strong predictor of of in-hospital mortality [18]. Higher in-hospital mortality was also seen among heart failure patients with increased plasma troponin-I levels. Additionally, Harvey et al. noted that serum troponins are commonly raised in acute exacerbations of COPD and appear to reflect the severity of the exacerbation[22]. Noninvasive ventilation (NIV) is a crucial strategy in treating the most severe acute COPD exacerbations. In this setting, release of Troponin-I at presentation, possibly reflecting cardiac strain resulting from respiratory insufficiency, may be an important predictor of patients who will require more aggressive treatment, such as NIV support.
Some of the demerits of this study are, it is a single centre study with small cohort size. We in this study also did not look at associated co-morbidities and their effect on short term prognosis. Long term follows up post exacerbation to assess hearth related quality of life could be included in future studies.
Conclusion
This study emphasizes the significant role of serum biomarkers, particularly BNP, Troponin-I, and CRP, in assessing outcomes in acute exacerbations of chronic obstructive pulmonary disease (AECOPD). The findings reveal that elevated BNP and Troponin-I levels at admission correlate strongly with in-hospital mortality, suggesting their utility as acute phase markers for respiratory and cardiac strain. While CRP and Fibrinogen reflect systemic inflammation, they showed limited predictive value for mortality in AECOPD. By incorporating BNP and Troponin-I into routine assessment, clinicians can more accurately stratify patient risk, guide intensive care decisions, and potentially improve outcomes. Future research, ideally involving larger, multi-centered studies, is warranted to further explore the integration of these biomarkers into clinical prediction models for optimizing COPD management.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of COPD. 2024 Report. Available from: www.goldcopd.org.
[2] Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non–smokers. Lancet. 2009; 374:733–743.
[3] Augusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2:367–370.
[4] Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007; 370:786–796.
[5] Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, et al. Evaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.
[6] Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000; 161:1608–1613.
[7] Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173:1114–1121.
[8] Papi A, Contoli M, Gaetano C, Mallia P, Johnston SL. Models of infection and exacerbations in COPD. Curr Opin Pharmacol. 2007; 7:259–265.
[9] Celli BR, Cote CG, Marin JM, Casanova C, de Oca MM, et al. The body–mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350:1005–1012.
[10] Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26:948–968.
[11] Chen YW, Leung JM, Sin DD. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One. 2016; 11:e0158843.
[12] Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjærg–Hansen A, et al. C–reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255.
[13] Helmy TA, Al–Masry AE, Ghazy HM. Role of inflammatory biomarkers in predicting mortality in COPD patients admitted to ICU. Egypt J Bronchol. 2014; 8:10–15.
[14] Stolz D, Christ–Crain M, Gencay MM, Bingisser R, Leuppi J, et al. Diagnostic value of signs, symptoms and inflammatory biomarkers in acute exacerbations of chronic obstructive pulmonary disease. Chest. 2007; 131:1058–1067.
[15] Shafiek H, Abd–elwahab N, Baddour M, Degady A, El–hoffy M et al. Outcome predictors of severe acute exacerbation of COPD: Role of inflammatory biomarkers. Int J Respir Pulm Med. 2016; 3:047.
[16] Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011; 66:764–768.
[17] Hawkins NM, Khosla A, Virani SA, McMurray JJ, FitzGerald JM. B–type natriuretic peptides in chronic obstructive pulmonary disease: a systematic review. BMC Pulm Med. 2017; 17:11.
[18] Baillard C, Fosse JP, Sebbane M, Chanques G, Vincent F, et al. Elevated cardiac troponin I is a strong predictor of in–hospital mortality in patients with acute exacerbation of COPD. Crit Care. 2011; 15:R116.
[19] Valvi D, Mannino DM, Müllerova H, Tal–Singer R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis. 2012; 7:173–182.
[20] Saldías PF, Díaz PO, Dreyse DJ, Gaggero BA, Sandoval Ac, et al. Etiology and biomarkers of systemic inflammation in mild to moderate COPD exacerbations. Rev Med Chil. 2012; 140:10–18.
[21] Manon JT, Lasse LL, Rank RS, Asser KM, Tal SR, et al. End product of fibrinogen is elevated in emphysematous chronic obstructive pulmonary disease and is predictive of mortality in the ECLIPSE cohor. Respirat Med. 2019; 160:105814.
[22] Elmenawi KA, Anil V, Gosal H, Kaur H, Ngassa HC, et al. The importance of measuring troponin in chronic obstructive pulmonary disease exacerbations: A systematic review. Cureus. 2021; 13:e17451.