Abstract
Background: WHO recommends linezolid (LZD) for Drug-Resistant Tuberculosis (DR-TB) despite its frequent adverse events, highlighting the importance of monitoring and managing adverse drug reactions (ADRs) to prevent unfavorable treatment outcomes. This study aims to assess the frequency, severity, predictors, and treatment impact of linezolid-related adverse events (AEs) in patients receiving bedaquiline-based all-oral longer regimens.
Materials and methods: Data was collected retrospectively from subjects diagnosed with DR-TB on LZD containing anti-TB drugs as per Programmatic Management of DR-TB (PMDT) guidelines-2021 at Nodal DR TB Centre - Mysore from January 2018 to January 2023.
Results: Among 182 subjects studied, total number of AEs recorded were 116. LZD related peripheral neuropathy accounted - 11%, optic neuritis - 10.4%, anaemia - 21.4% cases, nausea – 37.9% and vomiting - 18.7%. LZD was permanently discontinued because of AEs in 41/182 (35.3%) patients. The cumulative incidence of AEs increased rapidly during the first 6 months of treatment. These AEs resulted in LZD dose change or discontinuation in about one fourth (82/182, 45%) of all LZD treated patients. Patients with diabetes (DM) and People Living with HIV (PLHIV) associated with an increased risk of LZD related AEs. Patients with BMI < 18.5 were 1.7 times more likely to have LZD-related AEs.
Conclusion: Among the all MDR-TB patients treated with LZD containing regimen, >60% experienced LZD-related AEs. Haematological AEs were most common followed by peripheral and optic neuropathy of all LZD-related AEs. Patients with comorbid conditions like DM and PLHIV were significantly associated with AEs. Being underweight can be considered a potential risk factor for LZD-related AEs.
Keywords: drug resistant; tuberculosis; adverse events; linezolid
Full Text
Introduction
The growing incidence and diffusion of drug-resistant (DR) tuberculosis (TB) presents an enormous community health issue, imposing formidable challenges worldwide. Among these, Multidrug-Resistant TB (MDR-TB) and Rifampicin-Resistant TB (RR-TB) are big challenges in achieving successful treatment outcomes, requiring second line anti-TB drugs for its management [1]. WHO recommends linezolid (LZD) in treatment of drug-resistant tuberculosis (DRTB) despite its frequent association with adverse events [1].
Counselling of the patient and attenders regarding duration of the treatment, drugs used in the management and early identification of adverse events (AEs) should be done during pre-treatment evaluation. Patient should be enquired and evaluated at every follow-up for AEs, as well as treatment supporters should be involved in sensitization sessions for common AEs and timely referral to health care facility for further management. If the treatment is stopped in the field due to any reason, the Nodal/District Drug-Resistant Tuberculosis Centre (N/DDR- TBC) should be informed immediately to take the necessary action to reintroduce the treatment, modify or replace the regimen and/or manage the AEs N/DDR- TBC initiating the treatment should be immediately informed. All AEs should be notified and updated in NIKSHAY portal [2]. Monitoring adverse drug reactions (ADRs) is crucial in the treatment of DRTB. Failure to manage ADRs effectively can lead to several unfavourable outcomes [3]. Very few studies investigated the factors associated LZD-related AEs in MDR/XDR-TB patients.
The objectives of this study were to determine the frequency and severity of AEs attributed to LZD in patients undergoing treatment with a bedaquiline-based all oral longer regimen (AOLR), to identify demographic and clinical factors associated with an increased risk of LZD-related AEs, to assess how LZD-related AEs affect treatment adherence and completion rates, to evaluate their impact on treatment outcomes, including microbiological clearance and relapse rates post-treatment and to determine whether clinical characteristics can predict of adverse events associated with linezolid in the treatment of patients with MDR-TB.
Materials and methods
The present study was an institutional-based, retrospective study conducted at the Department of Respiratory Medicine, Princess Krishnajammanni TB and Chest Diseases Hospital (PKTBCDH), Mysore Medical College and Research Institute (MMCRI) in Mysore, from Jan 2019 to June 2024. Data for the study was collected during this period of 66 months who were diagnosed as cases of MDR/RR-TB receiving AOLR under PMDT after obtaining the Institutional Ethics Committee clearance certificate.
A self-designed data collection proforma was used. Recorded data included patient demographics such as age, gender, body mass index (BMI), diabetic status, HIV status, linezolid dose and frequency, and laboratory results such as white cell count, haemoglobin levels, platelet count. Anaemia was defined as haemoglobin for males < 13 g/dL, and for females < 12 g/dL, and categorized as mild (13-10g/dL males, 12-10g/dL in females), moderate (7-9.9g/dL), severe( below 7g/dL) [4]. Thrombocytopaenia was defined as low platelet levels with platelet <150000/mm3 [5]. Peripheral neuropathy was diagnosed using brief peripheral neuropathy screening tool and DAIDS criteria and categorised as mild, moderate and severe [6]. This was later confirmed with neurologist and nerve conduction studies. Optic neuritis was diagnosed with ophthalmologist consultation after performing colour vision test using Ischiara’s chart and fundoscopy using indirect ophthalmoscopy (IDO) by an ophthalmologist. Lactic acidosis was diagnosed with arterial blood gas using OPTI CCA-TS2 ABG analyser. The collected data were transferred onto a Microsoft Excel spreadsheet and analysed using standard computer programme SPSS for windows V 28.0. Significance level was fixed at 0.05 level.
Inclusion and exclusion criteria
All the diagnosed cases of MDR/RR-TB receiving a AOLR were included in the study. Subjects with preexisting peripheral neuropathy, preexisting severe anaemia, preexisting optic neuritis or known contraindications to LZD or other components of the treatment regimen, as identified through medical history or baseline assessments were excluded from the study.
Results
A total of 182 subjects were included in this study. Clinical characteristics of subjects receiving LZD containing regimen, and developed AEs are listed in Table 1. Among them 135 (74.1%) were males and 47 (25.82%) were females. Majority of them were underweight 74 (40.65%).
Table 1: Baseline characteristics of subjects who received linezolid.
|
Base line characteristics
|
Side effects of linezolid
|
|
No
|
Yes
|
|
Count
|
Row N %
|
Count
|
Row N %
|
|
Gender
|
Male
|
52
|
38.5%
|
83
|
61.5%
|
|
Female
|
14
|
29.8%
|
33
|
70.2%
|
|
TG
|
0
|
0.0%
|
0
|
0.0%
|
|
BMI
|
<18.5
|
53
|
41.7%
|
74
|
58.3%
|
|
18.5 -24.9
|
11
|
23.4%
|
36
|
76.6%
|
|
25 - 29.9
|
2
|
25.0%
|
6
|
75.0%
|
|
30 - 34.9
|
0
|
0.0%
|
0
|
0.0%
|
|
35-39.9
|
0
|
0.0%
|
0
|
0.0%
|
|
>40
|
0
|
0.0%
|
0
|
0.0%
|
|
DM
|
Yes
|
6
|
14.6%
|
35
|
85.4%
|
|
No
|
60
|
42.6%
|
81
|
57.4%
|
|
PLWHAIDS
|
Yes
|
0
|
0.0%
|
20
|
100.0%
|
|
No
|
66
|
40.7%
|
96
|
59.3%
|
|
Other comorbidities
|
No
|
56
|
36.4%
|
98
|
63.6%
|
|
Yes
|
10
|
35.7%
|
18
|
64.3%
|
|
Deranged RFT
|
No
|
66
|
36.3%
|
116
|
63.7%
|
|
Yes
|
0
|
0.0%
|
0
|
0.0%
|
|
Deranged LFT
|
No
|
66
|
36.5%
|
115
|
63.5%
|
|
Yes
|
0
|
0.0%
|
1
|
100.0%
|
|
Prior TB RX
|
No
|
26
|
35.1%
|
48
|
64.9%
|
|
Yes
|
40
|
37.0%
|
68
|
63.0%
|
|
Cavity on CXR
|
Yes
|
38
|
40.9%
|
55
|
59.1%
|
|
No
|
28
|
31.5%
|
61
|
68.5%
|
|
Type of DRTB
|
RR
|
30
|
38.0%
|
49
|
62.0%
|
|
MDR
|
17
|
33.3%
|
34
|
66.7%
|
|
Pre XDR
|
18
|
36.0%
|
32
|
64.0%
|
|
XDR
|
0
|
0.0%
|
0
|
0.0%
|
|
Poly drug
|
1
|
50.0%
|
1
|
50.0%
|
In this study 116 AEs were observed. Among total number of AEs, the proportion of LZD related peripheral neuropathy was 11% (20) among which 4.9% had moderate grade, 6 % hade severe grade. Optic neuritis was observed in 10.4% (19), anaemia in 21.4% (39), among which 6% hade moderate anaemia and 15.4% had severe anaemia. Nausea and vomiting were observed in 37.9% (69) and 18.7% (34) respectively. Lactic acidosis was observed in 6.6% (12). The frequency and severity of AEs attributed to LZD are depicted in Figure 1.
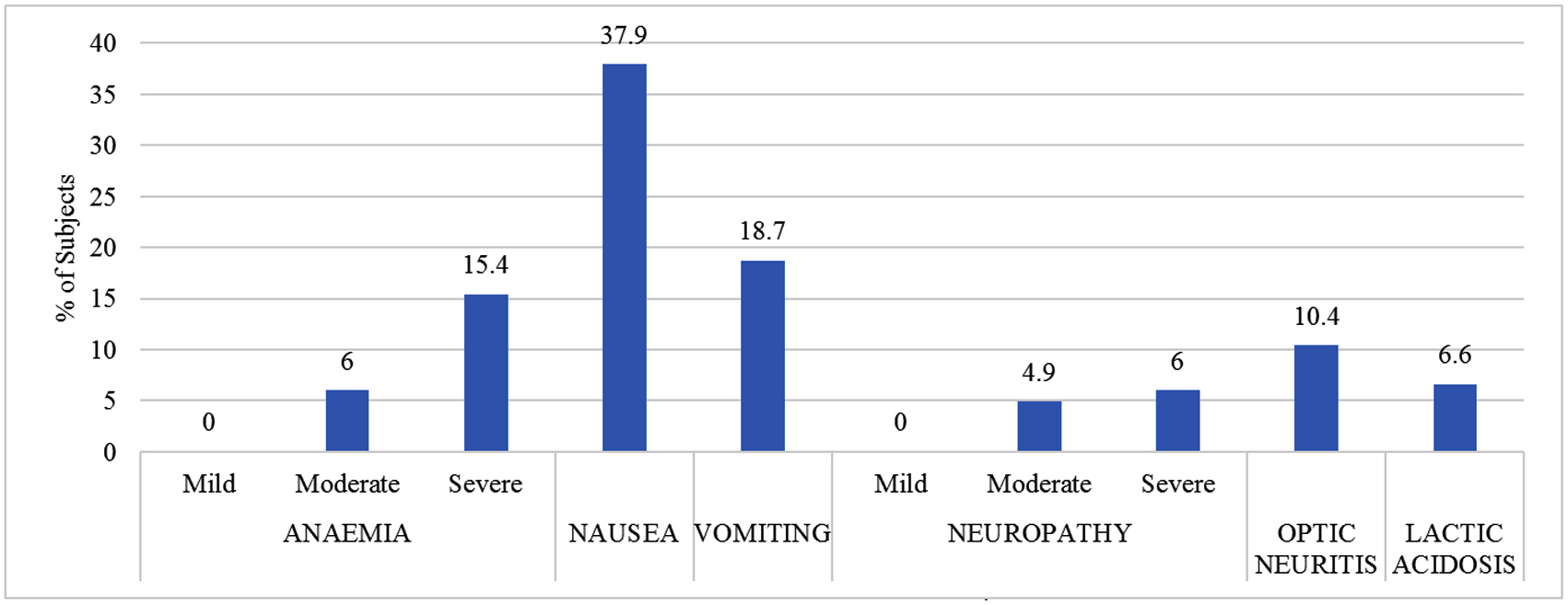
Figure 1: Frequency and severity of AEs attributed to LZD.
Demographic and clinical factors associated with an increased risk of LZD related AEs are DM (P value- 0.001) and PLHIV( P value- 0.000). Table 2 shows significant factors associated with AEs of LZD. All the patients who were known case of PLHIV (20/182) had AEs. 85.37% (35/182) of diabetic patients had AEs. The cumulative incidence of AEs observed during the first 6 months of treatment. Within 1 month of treatment 48 (41.38%) had developed AEs. From 2nd month to 6th month of treatment 56 (48.28%) patients had developed AEs. After 6 months of LZD, 12 (10.34%) had developed AEs is shown in Table 3.
Table 2: Characteristics of subjects receiving LZD containing regimen, and developed AEs.
|
Age (mean ± SD), years
|
37±14.5
|
|
Gender
|
|
|
Male
|
83 (61.5%)
|
|
|
Female
|
33 (70.2%)
|
|
BMI (< 18.5%)
|
74 (58.3%)
|
|
Comorbidities
|
|
|
Type 2 DM
|
35 (85.4%)
|
|
|
PLHIV
|
20 (100%)
|
|
|
Prior history of TB treatment
|
68 (63.0%)
|
|
|
Cavity on CXR
|
55 (59.1%)
|
|
Type of DRTB
|
|
|
RR TB
|
49 (62%)
|
|
|
MDR TB
|
34 (66.7%)
|
|
|
PRE XDR TB
|
32 (64%)
|
|
|
XDR TB
|
0 (0%)
|
|
|
POLY DRUG TB
|
1 (50%)
|
Abbreviations: BMI- Body-Mass Index, DM - diabetes mellitus; PLHIV - People Living With Human Immunodeficiency Virus; CXR- Chest X Ray; TB- tuberculosis; RR-TB - Rifampicin-Resistant Tuberculosis; MDR-TB - Multidrug-Resistant Tuberculosis; pre-XDR – pre-Extensively Drug-Resistant Tuberculosis; XDR-TB- Extensively Drug-Resistant Tuberculosis.
These AEs resulted in LZD dose change to 300 mg in 40 (34.5%) patients and discontinuation in (40/182, 31.3%) of all LZD treated patients. LZD was temporarily stopped in 35 (30.2%) patients until AEs were resolved. The impact on LZD dosing shown in Figure 2. Patients who had LZD-related AEs had more cure rates and less death rates compared to those who didn’t suffer with any of the AEs. LZD-related AEs impact on treatment outcomes listed in Tables 3-5.
Table 3: Significant factors associated with AEs of LZD.
|
Factors
|
P value
|
|
DM
|
0.001
|
|
PLHIV
|
0.000
|
Abbreviations: DM - diabetes mellitus; PLHIV - People Living with Human Immunodeficiency Virus; BMI- Body-Mass Index.
Table 4: Duration to develop AE’s with LZD.
|
Duration
|
Number
|
%
|
|
<1 month
|
48
|
26.4%
|
|
1-6 months
|
56
|
30.8%
|
|
> 6 months
|
12
|
6.6%
|
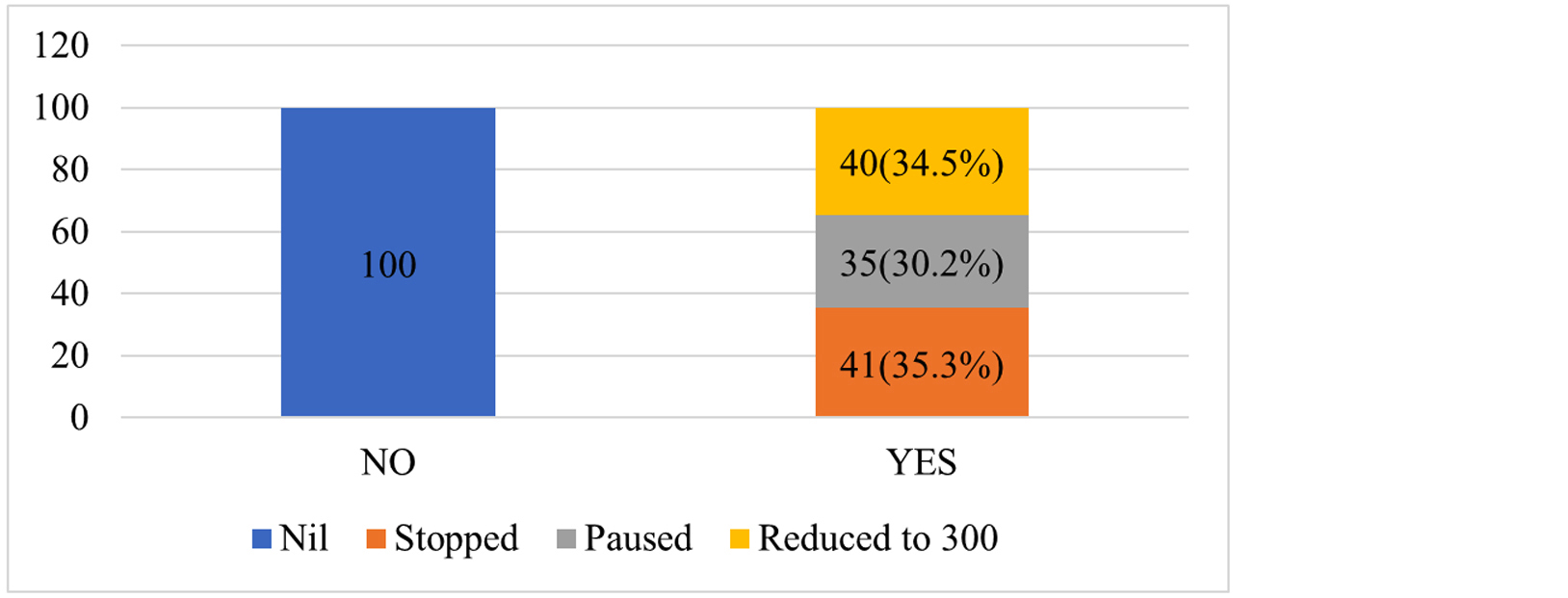
Figure 2: Stacked bar percent of any AE of LZD by impact on LZD dosing.
Table 5: LZD-related AEs impact on treatment outcomes.
|
|
Cured
|
Treatment Completed
|
On Rx
|
Death
|
LFU
|
|
n
|
%
|
N
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
|
Side effects of LZD
|
No
|
11
|
16.7%
|
19
|
28.8%
|
2
|
3.0%
|
26
|
39.4%
|
8
|
12.1%
|
|
Yes
|
28
|
24.1%
|
39
|
33.6%
|
12
|
10.3%
|
30
|
25.9%
|
7
|
6.0%
|
Abbreviations: LZD - linezolid; On Rx- on treatment; LFU- Lost to Follow Up.
Discussions
Out of 182 patients included in our study, the most frequent AEs attributed to LZD includes gastrointestinal (nausea 37.9% and vomiting 18.7%) followed by anaemia (21.4%), peripheral neuropathy (11%) and optic neuritis (10.4%). Univariate analysis was conducted to see whether clinical factors are associated with increased risk of LZD related AEs.
Patients with BMI <18.5 were 1.7 times more likely to have LZD related AEs. This finding was consistent with the study conducted by Oehadian et al [7] in which patients with BMI were 2.6 times more likely to develop haematological AEs.
Patients with DM and PLHIV were significantly associated with LZD related AEs. In this study the occurrence of LZD related AE was 63.7% among which 21.4% had haematological AEs which included moderate and severe anaemia. The prevalence of haematological AEs is almost similar to the study conducted by Letswee et al [8] in which 25.9% had haematological AEs were PLHIV. Where as in study conducted by Mishra et al. Peripheral neuropathy was the most frequent linezolid-ADR followed by lactic acidosis and anemia respectively [9].
The prevalence of non-haematological AEs was 21.4% of these 11% had peripheral neuropathy and 10.4% had optic neuritis. The prevalence of LZD associated neuropathy was lower than previous study conducted by Zhang et al [10] where neuropathy was observed in 40% of patients out of which 69% peripheral neuropathy, 24% had optic neuritis, and 7% had both.
The cumulative incidence of AEs increased rapidly during first 6 months in 56 (48.28%) patients. Patients developed adverse effect from 2nd to 6th month of treatment. This observation was consistent and similar to the study conducted by Imperial et al [11] where LZD related neuropathy occurred after 3 to 6 months of treatment.
These AEs resulted in LZD dose change or discontinuation in (44%) of all LZD treated patients and among those patients in whom dose was reduced to 300mg the treatment outcome was better with cure rate of 22.5% and treatment completion rate in 40% which is better when compared to those continued taking 600mg of LZD (Table 6 and Figure 3). This was found consistent with previous study by Anjeli Mase et al [12] where patients on standard dose LZD were compared with low dose 300 mg or intermittent dosing patients and observed that those on low-dose LZD experienced significantly fewer AEs with better treatment outcomes. Johanna Kuhlin et al found that patients who had taken LZD dose ≥12 mg/kg/d was associated with AEs and regular follow-up for AEs and adjusting dosing according to body weight followed-up by monitoring of drug concentration may reduce toxicity [13].
Table 6: Impact of linezolid dose modification on outcome.
|
|
N
|
Cured
|
Treatment completed
|
On treatment
|
Excluded
|
Death
|
LFU
|
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
n
|
%
|
|
Impact on linezolid Dose modification
|
Nil
|
65
|
10
|
15.4%
|
19
|
29.2%
|
2
|
3.1%
|
0
|
0.0%
|
26
|
40.0%
|
8
|
12.3%
|
|
Reduced to 300
|
40
|
9
|
22.5%
|
16
|
40.0%
|
2
|
5.0%
|
0
|
0.0%
|
12
|
30.0%
|
1
|
2.5%
|
|
Paused
|
35
|
10
|
28.6%
|
10
|
28.6%
|
3
|
8.6%
|
0
|
0.0%
|
8
|
22.9%
|
4
|
11.4%
|
|
Stopped
|
42
|
10
|
23.8%
|
13
|
31.0%
|
7
|
16.7%
|
0
|
0.0%
|
10
|
23.8%
|
2
|
4.8%
|
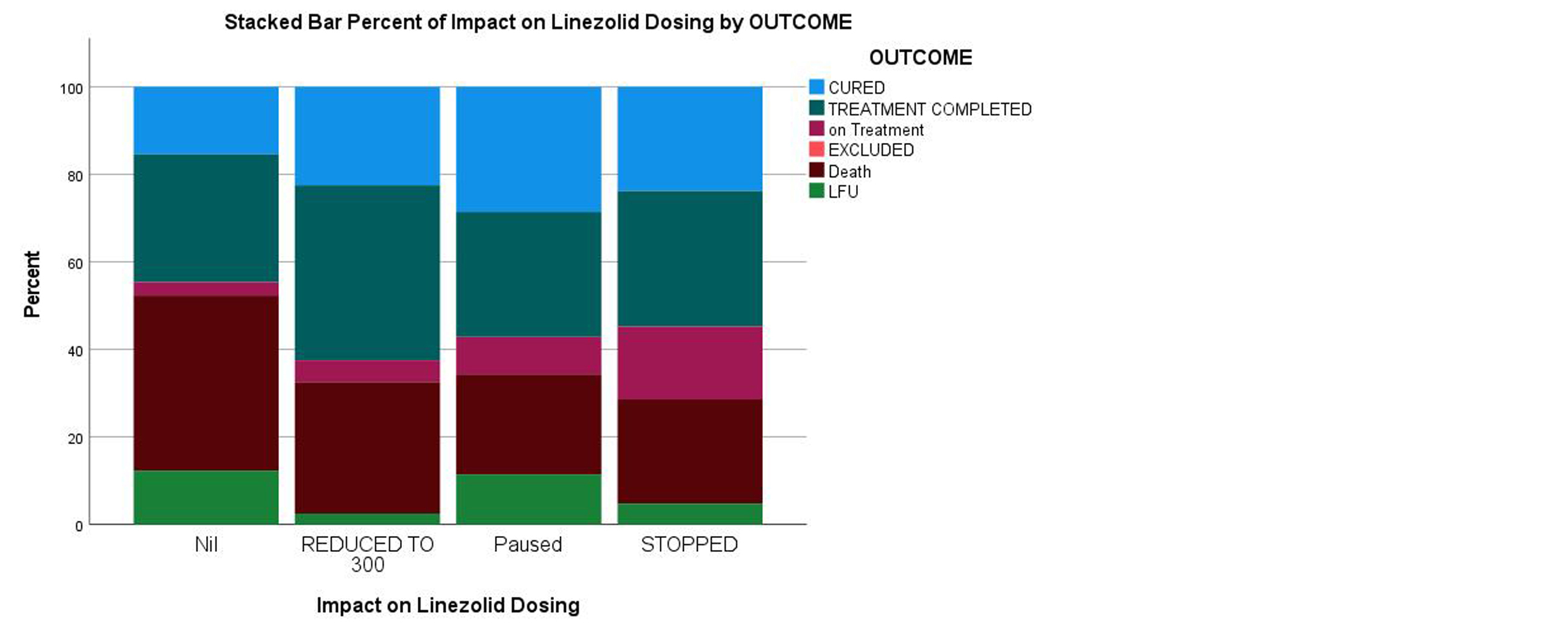
Figure 3: Stacked bar percent of linezolid dose modification and treatment outcomes.
Limitations: A significant number of patients (12) had not completed treatment, which could result in an underestimation of LZD-related AEs. Gastrointestinal symptoms can be attributed to any other ATT. The study findings indicate that risk factors identified are for all AES and it might differ for haematological and non-haematological LZD-related AEs.
Conclusions
This study highlights that more than 60% of MDR-TB patients receiving a linezolid-containing regimen experienced adverse events (AEs), underscoring the need for vigilant monitoring. Comorbid conditions such as diabetes and HIV infection were significantly associated with an increased risk of LZD-related AEs, while being underweight also emerged as a potential predictor. However, a reduced linezolid dose of 300 mg was associated with improved treatment completion and cure rates compared to the standard 600 mg dose. These findings suggest that individualized dosing strategies and early identification of high-risk patients may improve treatment tolerability and outcomes in DR-TB management. Acknowledgements We thank all the physicians involved in the management and care of our patients, particularly the ophthalmic team, and neurology team for their professional opinions. District TB Officer- Mysore for supporting the study.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] World Health Organization. Global Tuberculosis Report 2022. Geneva: WHO; 2023.
[2] National Guidelines for Management of Drug Resistant TB. November 2024.
[3] Schaaf HS, Garcia-Prats AJ, Hesseling AC, Seddon JA. Managing multidrug-resistant tuberculosis in children: review of recent developments. Curr Opin Infect Dis. 2014; 27:211–9.
[4] World Health Organization. Guideline on haemoglobin cutoffs to define anaemia in individuals and populations. Geneva: WHO.
[5] Erkurt MA, Kaya E, Berber I, Koroglu M, Kuku I. Thrombocytopenia in adults. J Hematol. 2012; 1:44–53.
[6] National Guidelines for Management of Drug-Resistant TB. November 2024.
[7] Oehadian A, Bastos ML, Centis R, D'Ambrosio L, Migliori GB, et al. Occurrence and predictors of adverse events associated with linezolid in the treatment of patients with MDR-TB. Pulmonology. 2024; 30:184–187.
[8] Letswee G, Kamau H, Gaida R, Truter I. Haematological adverse effects associated with linezolid in patients with drug-resistant tuberculosis: an exploratory study. Int J Pharm Pract. 2019; 27:575–577.
[9] Mishra G, Alffenaar JW, Munje R, Khateeb S. Adverse drug reactions due to linezolid in the programmatic management of drug-resistant tuberculosis in India: a retrospective multicenter study. Indian J Tuberc. 2024; 71:S101–S109.
[10] Zhang P, Li W, Liu M, Zhan S, Zhang H, et al. Linezolid-associated neuropathy in patients with MDR/XDR tuberculosis in Shenzhen, China. Infect Drug Resist. 2022; 15:2617–2624.
[11] Imperial MZ, Nedelman JR, Conradie F, Savic RM. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin Infect Dis. 2022; 74:1736–1747.
[12] Mase A, Lowenthal P, True L, Henry L, Barry P, et al. Low-dose linezolid for treatment of patients with multidrug-resistant tuberculosis. Open Forum Infect Dis. 2022; 9:ofac500.
[13] Kuhlin J, Forsman LD, Osman A, Skagerberg M, Jonsson J, et al. Increased risk of adverse drug reactions by higher linezolid dose per weight in multidrug-resistant tuberculosis. Int J Antimicrob Agents. 2024; 64:107302.