Full Text
Introduction
COVID-19 is a respiratory pathogenic strain that contributed to an estimate of 29% of expected all cause deaths from June 2020 to July 2021 in India [1]. Fatalities often follow severe systemic infections which may be present as ARDS (Acute Respiratory Distress Syndrome), Shock and Coagulation abnormalities associated with DIC (Disseminated Intravascular Coagulation), and Thrombotic Microangiopathy [2,3]. Subsequent risk for thrombosis was recognized by the American Society of Hematology (ASH) which made recommendations for administration of Venous thromboembolism (VTE) thromboprophylaxis with LMWH (Low molecular weight heparin) or Fondaparinux to all hospitalized patients [4].
Comorbidities such as diabetes mellitus, hypertension and cardiovascular diseases are hypercoagulable states which may increase the risk of mortality. Literature indicates that the mortality of approximately 80 percent of individuals with diabetes mellitus is through hypercoagulability induced thrombosis. These may occur in the form of cardiovascular events or stroke. This may be accounted to the underlying physiology of dysfunctional coagulation cascade, comprising of elevated coagulation activation markers such as thrombin - antithrombin complexes and elevated clotting factors such as fibrinogen, von Willebrand factor, factor VII, factor VIII, factor XI and factor XII which may play an important role in the coagulation pathway [5].
Hypertension on the other hand, causes an imbalance of cytokines such as IL-6, IL-7 and TNF. Moreover, abnormalities of vessel walls, abnormal levels of haemostatic factors, increased risk of fibrinolysis and altered blood flow may produce a hypercoagulable state [6, 7]. In the case of cardiovascular diseases, an increased level of inflammatory cytokines which stimulate procoagulant activation and hemodynamic instability may produce a hypercoagulable state while also posing an additional risk of ischemia and thrombosis [8].
In this study, we compared the blood parameters that affect coagulation between comorbid and non-comorbid individuals infected with COVID-19, so as to study the variations between the two groups and to assess the effect of comorbidities on individuals affected with COVID-19. This study was devised in the view that the hypercoagulable states of pre-existing comorbidities may pose an additional burden and increased risk of coagulopathy amid the already hypercoagulable state induced by COVID-19 infection.
Method
A retrospective study was conducted at PSG Institute of Medical Sciences and Research, Coimbatore on approval from the Institutional Ethics Committee Board. Inpatient lists and case sheets of all patients admitted to PSG Hospitals between November 2020 to November 2021 were collected and data was analyzed. The study was conducted along the lines of the ‘Institutional Health Insurance Portability and Accountability Act (HIPAA)’ of PSG Institute of Medical Sciences and Research, thus ensuring that Patient Protected health information (PHI) was not collected nor documented.
In our study, 2 broad groups of individuals with COVID-19 were considered. Group 1 consisted of individuals without comorbidities and Group 2 consisted of Individuals with comorbidities. Both males and females between the age groups of 20-60 years who were diagnosed with COVID-19 were included in the study. Only Diabetes, Hypertension and Cardiovascular disease were considered to be comorbidities. Elderly above the age of 70, bedridden patients (prior to the onset of COVID-19), Pregnancy, End stage cardiovascular disease, individuals with other coexisting comorbidities such as COPD or CKD, underlying malignancies and those previously under palliative care were excluded from the study. Coagulation profiles, specifically platelets, D-dimer and other parameters that may influence the coagulation profile such as NLR ratio were considered in the study. Moreover, the absolute neutrophil count, absolute lymphocyte count and hemoglobin were also analyzed and subjected to comparison.
Following exclusion, 264 patients were selected (120 comorbid individuals and 144 non-comorbid individuals) out of a total of 460 patient records. Patient characteristics such as Age, Gender and comorbidities were recorded. Demographic and laboratory data were collected and subjected to statistical analysis. An unpaired t-test was performed using SPSS software version 28. P value < 0.05 was considered to be significant in the study.
Results
A total of 460 patients were admitted and underwent treatment at PSG hospitals between November 2020 to November 2021. Following exclusion, 264 patient’s records were selected which consisted of 120 comorbid individuals (70 males and 51 females) and 144 non-comorbid individuals (62 males and 88 females). The mean age among individuals with comorbidities was 54.96 years and non-comorbid individuals was 40.62 years. Among the 120 comorbid individuals, 65 individuals presented with diabetes mellitus, 75 individuals presented with hypertension and 22 individuals presented with coronary artery disease. A small proportion of patients within the initial sample size (460 patients), presented with asthma, COPD and bronchiectasis. However, they were excluded from the study due to insignificant samples and missing data. Figure 1 shows the age distribution of the comorbid and non-comorbid population, Figure 2 depicts the distribution of males and females within the two broad groups and Figure 3 depicts the distribution of comorbidities in the study population.
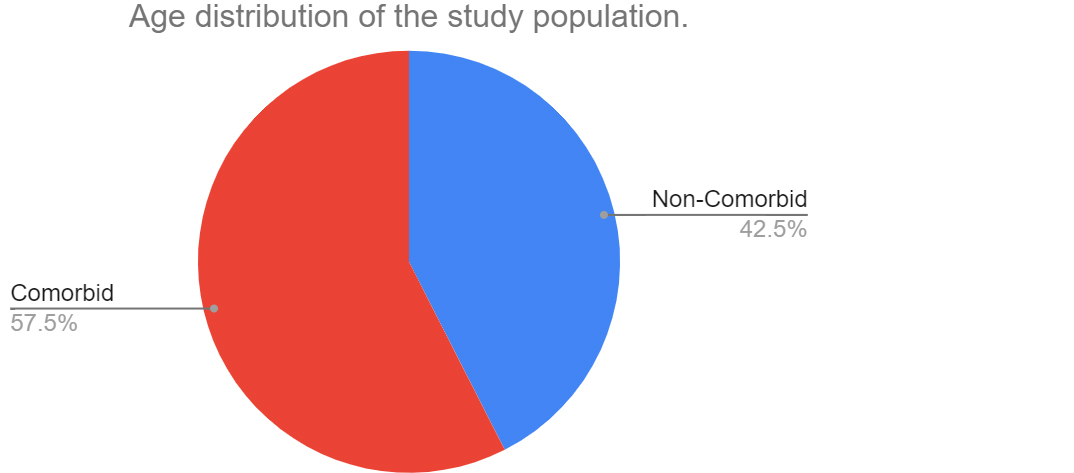
Figure 1: Age distribution of the comorbid and non-comorbid population.
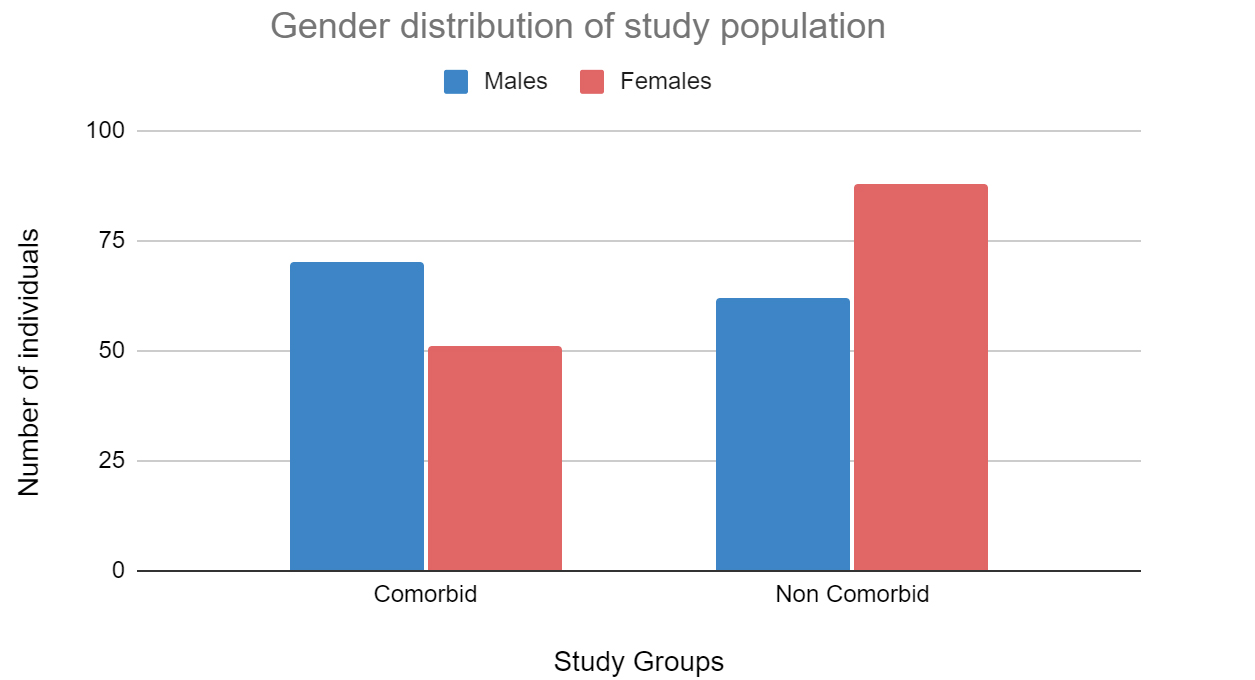
Figure 2: Distribution of males and females within the two broad groups.
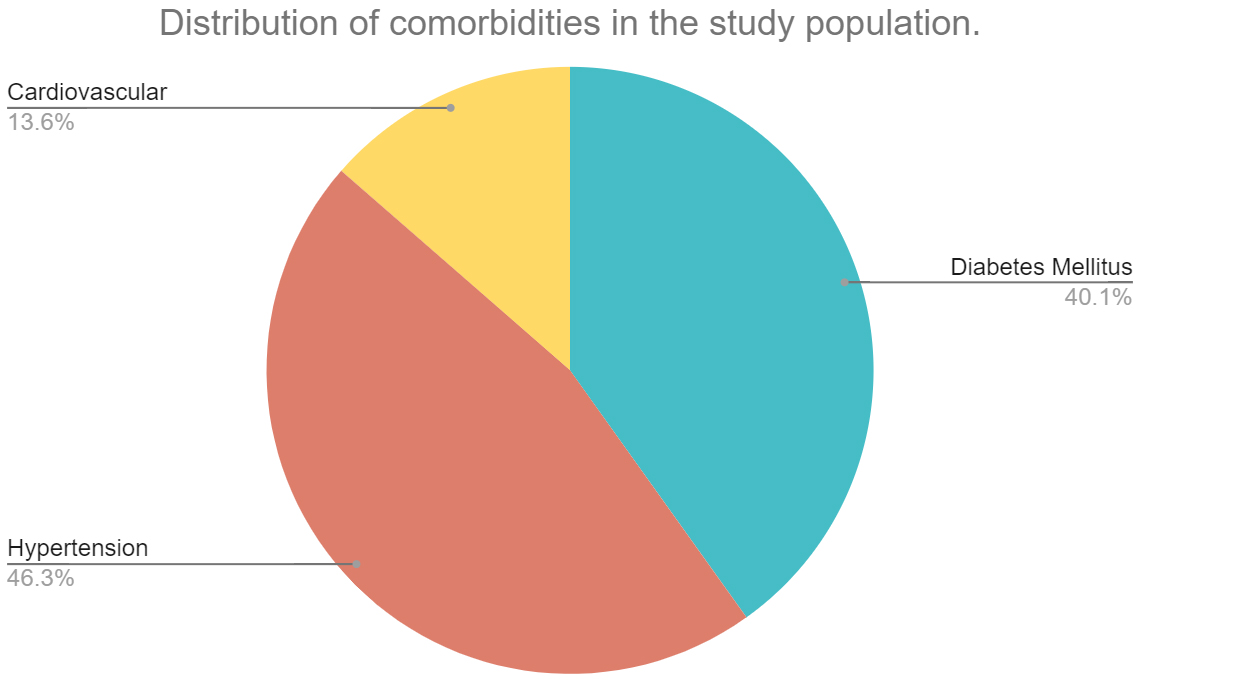
Figure 3: Distribution of comorbidities in the study population.
In this study, we considered platelets, d-dimer and NLR ratio as blood coagulation parameters. While the NLR ratio (neutrophil / lymphocyte ratio) might not be directly indicative of the coagulation, studies have shown a positive association between the NLR ratio and coagulation indexes, namely PT and APTT.
Table 1 describes the data analysis of comorbid and non-comorbid patient data with respect to these coagulation parameters, absolute neutrophil and lymphocyte count and hemoglobin. The absolute neutrophil count and NLR ratio was statistically significant between the two groups of individuals. Figure 4a compares the platelet counts and Figure 4b compares the other study parameters between the two groups of individuals.
Table 1: Data analysis of comorbid and non - comorbid individuals with respect to the corresponding parameters.
|
Parameter
|
Group
N (non-comorbid) = 144
N (comorbid) = 120
|
Mean +/- SD
|
p value
|
|
Platelets
(x 10^3/uL)
|
Non - comorbid individuals
|
239.69 +/- 70.83
|
0.506
|
|
Comorbid individuals
|
233.08 +/- 87.15
|
|
D - dimer
(mg/L)
|
Non - comorbid individuals
|
1.43 +/- 3.70
|
0.973
|
|
Comorbid individuals
|
1.41 +/- 3.22
|
|
Absolute neutrophil count
(x 10^3/uL)
|
Non - comorbid individuals
|
4.01 +/- 3
|
0.016**
|
|
Comorbid individuals
|
4.90 +/- 3.03
|
|
Absolute Lymphocyte count
(x 10^3/uL)
|
Non - comorbid individuals
|
1.56 +/- 0.67
|
0.425
|
|
Comorbid individuals
|
1.49 +/- 0.87
|
|
NLR ratio
|
Non - comorbid individuals
|
3.37 +/- 4.21
|
0.038**
|
|
Comorbid individuals
|
4.22 +/- 3.43
|
|
Hemoglobin
(g/L)
|
Non - comorbid individuals
|
12.39 +/- 1.94
|
0.534
|
|
Comorbid individuals
|
12.24 +/- 1.93
|
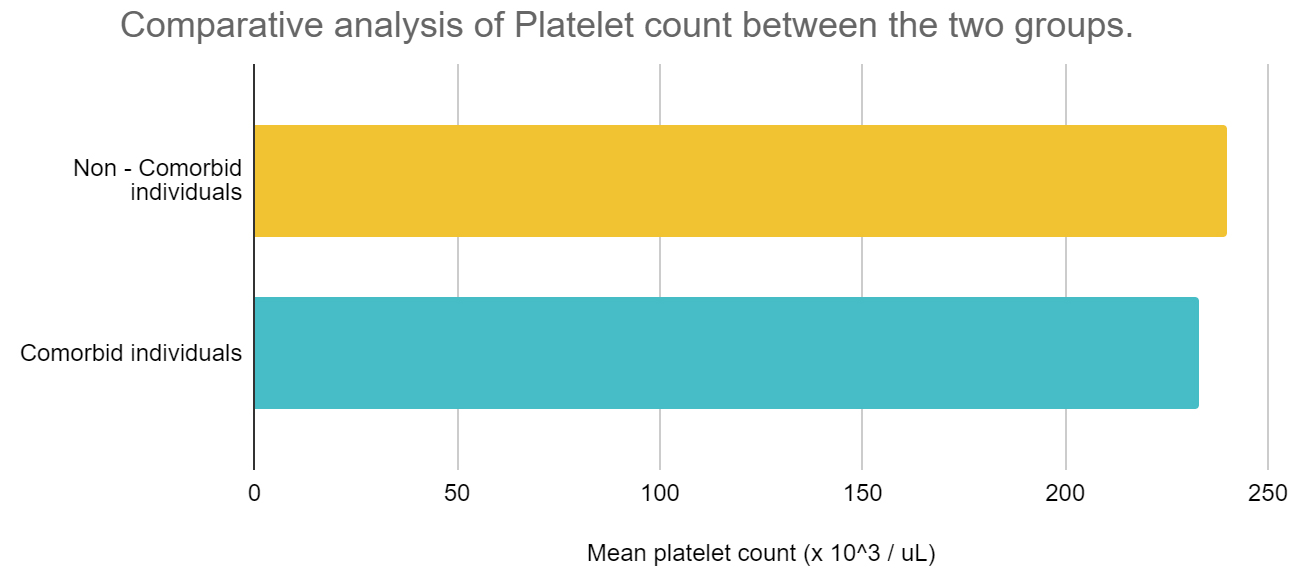
Figure 4a: Comparison of platelet counts between the two groups of individuals.
Abbreviations: Yellow bar diagrams – Non-comorbid individuals; Blue bar diagrams - Comorbid individuals.
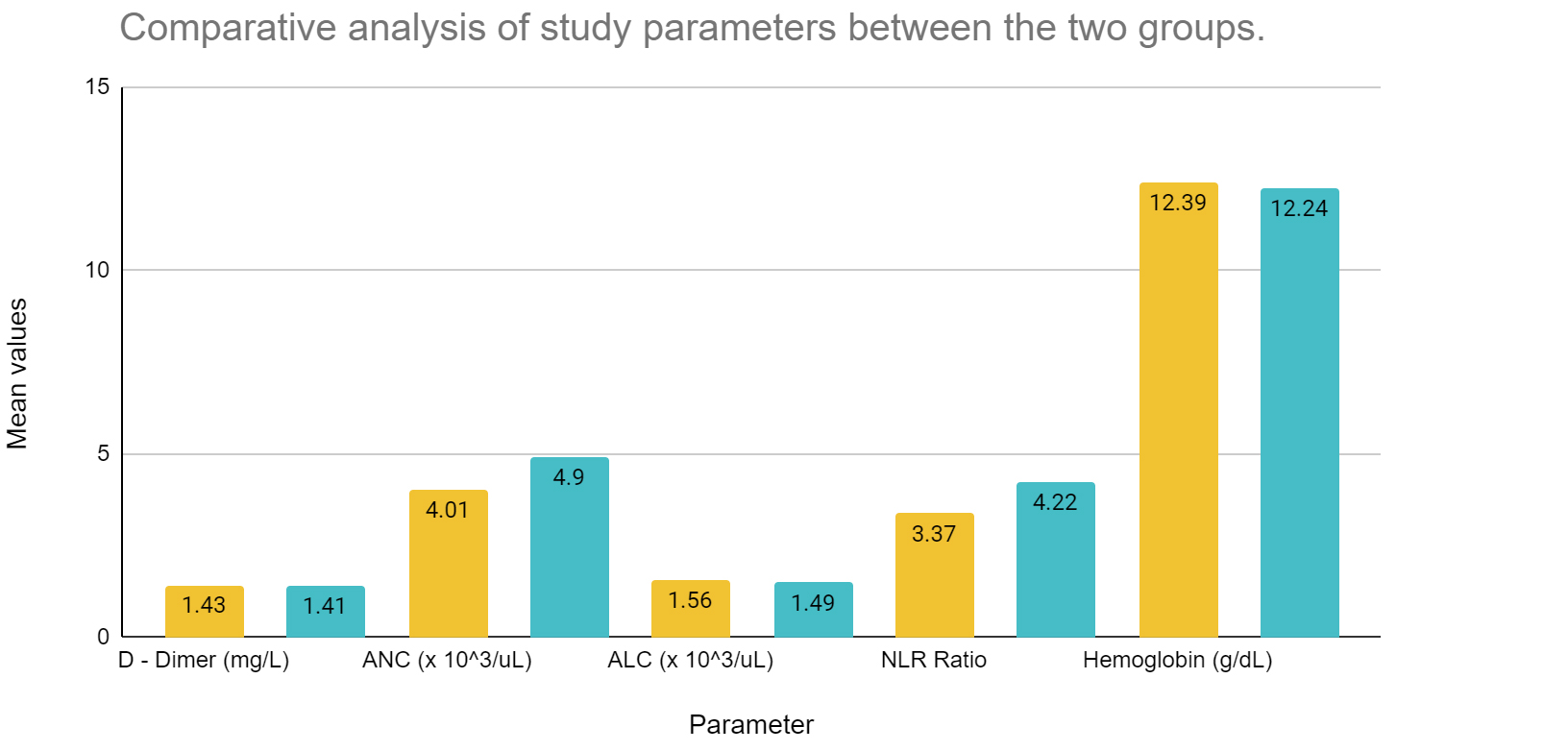
Figure 4b: Comparison of study parameters between the two groups of individuals.
Abbreviations: Yellow bar diagrams – Non-comorbid individuals; Blue bar diagrams - Comorbid individuals; ANC - Absolute neutrophil count; ALC - Absolute lymphocyte count; NLR - Neutrophil lymphocyte ratio.
Coagulation parameters, namely platelet count is increased amid non comorbid individuals, however, the NLR ratio seems to be increased among those with comorbidities. D-dimer shows minor differences between the two sets of population. Absolute neutrophil count is increased among those with comorbidities, however, absolute lymphocyte count is more or less similar between the two groups. The population averages of hemoglobin varied between 12.39 and 12.24 g/dL between the two groups, thereby on broader terms, we can state that no significant anemia was present among the two population groups, which may alter the coagulation profile in hospitalized COVID-19 individuals.
Discussion
Coagulopathy in COVID-19 is a concerning factor in view of intravascular coagulation which may increase the risk for mortality. Similarly, dysfunctional coagulation cascades in diabetes mellitus and increased risks for coagulopathy amid those with hypertension and cardiovascular diseases may aggravate the risk for mortality in the setting of an already hypercoagulable state induced by COVID-19 infection. In this study, we have tried to evaluate the parameters which indicate coagulation among comorbid and non-comorbid individuals with COVID-19, so as to note if any significant variations are prevalent among these parameters which may indicate an increased risk for coagulopathy.
Increased D-dimer and fibrinogen levels, decreased platelet count and prolonged prothrombin time were commonly seen amid COVID-19 patients with Coagulopathy. The study conducted by Levi M, et al., also discussed the increased risk of mortality amid patients with elevated D-dimer levels and the possibility of DIC (Disseminated intravascular coagulation) among those with increased D-dimer levels, mild thrombocytopenia and prolonged prothrombin time [9]. Similar studies showed elevated FDP, D-Dimer levels, prolonged aPTT and PT-INR and a significant correlation between coagulation dysfunction and prognosis amid individuals presenting with a severe COVID-19 infection [10]. In our study, D-dimer levels were elevated in most patients, possibly indicating an increased risk of coagulopathy, although platelet count remained normal. Moreover, no significant associations of d-dimer levels or platelet counts were present between those with and without comorbidities. This was consistent with the study conducted by Yujing Sun, et al on the diabetic population infected with COVID-19. The study noted a relative increase in D-dimer levels, Fibrinogen and FDP among individuals with diabetes, however, no statistically significant association was found between the 2 groups in terms of coagulation function [11]. However, a study in the Chinese population depicted an eightfold increase in the risk of mortality among COVID-19 individuals with diabetes as compared to those without pre-existing mortalities [12], however, the role of coagulopathy in the increased risk of mortality was not known.
Studies conducted among individuals with pre-existing cardiovascular diseases showed significant risk of Cardiac Injury and subsequent mortality likely to be a result of viral hypercoagulable state and DIC (Disseminated Intravascular coagulation) [13]. This may be a result of increased levels of inflammatory cytokines which may stimulate procoagulant activation and hemodynamic instability, thereby posing an additional risk of ischemia and thrombosis [8]. A nationwide study conducted on the Swedish population on the association between COVID-19 and acute cardiovascular events showed a fivefold increase on the incidence of acute myocardial infarction and a tenfold increase in the incidence of ischemic stroke following COVID-19. Moreover, the study also showed an increased prevalence of atrial fibrillation among individuals with COVID-19, which could have induced stroke [14]. Another study conducted by Zahra RE, et al., also noted increased rates of heart failure and pericarditis amid hospitalized individuals [15]. However, both these studies considered incident cardiovascular outcomes among non-comorbid individuals, thereby an underestimation of the risk for adverse cardiovascular events may be present.
Hypertension is also known to cause hypercoagulability and an increased risk of mortality among those diagnosed with COVID-19. This could be attributed to the imbalance of cytokines such as IL-6, IL-7 and TNF [6]. A study conducted by Wang, et al., noted an increased ICU admission of hypertensive individuals with COVID-19 infection [16]. Similarly, another study indicated an increased hazard ratio and possibility of severe COVID-19 outcomes amid those with pre-existing hypertension [17]. While literature indicates the outcomes, mortality and risks for coagulopathy with comorbidities, to the best of our knowledge, there has been little to no research which studied the variations in coagulation profile in individuals with hypertension, diabetes or cardiovascular diseases.
In our study, we have studied the variations in platelets, d-dimer and NLR ratio (neutrophil / lymphocyte ratio) as indicative of coagulopathy. Literature showed the NLR ratio to have positive associations with PT and APTT levels [18]. Hence, an increased NLR ratio and its significant association between comorbid and non-comorbid individuals might indicate an increased risk of coagulation amid individuals with comorbidities. Similarly, the absolute neutrophil count was elevated and showed significant associations between the two groups. This is consistent with the studies that show a correlation between increased neutrophil levels and/or NLR ratio with the severity of COVID-19 [19, 20]. Li J, et al., additionally explored the role of low-density neutrophils (LDN) and neutrophil extracellular traps (NET), thereby emphasising the key role of neutrophils in the severity of COVID-19 [20]. It is thus possible that an increased neutrophil count may indirectly be an indicator of the severity of COVID-19 among individuals with comorbidities, given its strong correlation with COVID-19 among the healthy population.
The average hemoglobin levels in our population were within normal limits, likely indicative that anemia did not play a role in the worsening of the individuals. Moreover, it may also be suggestive of the population distribution, wherein, critically ill COVID-19 patients might not have been considered in whom anemia is a common phenomenon.
Limitations: Our study was a single-center, tertiary care hospital study conducted at PSG Hospitals. It was designed to meet the calculated minimum sample size, ensuring statistical relevance for the data. However, the limited number of cases and regional population differences make the study inadequate for drawing conclusive results applicable to a larger population. Consequently, a multicentric study with a larger sample size and greater regional diversity would likely provide more precise results, as the data from a tertiary care setting may not accurately reflect the disease profile of the broader community.
Conclusion
Blood parameters which influence coagulation were studied among comorbid and non-comorbid individuals. NLR ratio and absolute neutrophil count was elevated among those with comorbidities as compared to those without comorbidities. Moreover, they showed a significant association between the two groups of individuals. While these may be suggestive of increased risk for coagulation, platelet count was normal and d-dimer levels were consistent and showed an insignificant association between the two groups. Hence, further research is essential to study the possible risk of coagulopathy amid infected individuals with comorbidities.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Jha P, Deshmukh Y, Tumbe C, Suraweera W, Bhowmick A, et al. COVID mortality in India: National survey data and health facility deaths. Science. 2022; 375:667–671.
[2] Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, et al. Covid-19 in critically ill patients in the seattle region - case series. New England Journal of Medicine. 2020; 382:2012–2022.
[3] Levi M, Scully M. How I treat disseminated intravascular coagulation. Blood. 2018; 131:845–854.
[4] Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020; 135:2033–2040.
[5] Carr ME. Diabetes mellitus. J Diabet Complicat. 2001; 15:44–54.
[6] Huang S, Wang J, Liu F, Liu J, Cao G, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertension Research. 2020; 43:824–831.
[7] Lip GY, Li-Saw-Hee FL. Does hypertension confer a hypercoagulable state? J Hypertens. 1998; 16:913–916.
[8] Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020; 13:1833–1839.
[9] Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematology. 2020; 7:e438–e440.
[10] Zhang Y, He L, Chen H, Lu S, Xiong Y, et al. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients: Retrospective analysis. Int J Lab Hematol. 2020; 42:766–772.
[11] Sun Y, Zhao R, Hu Z, Wang W, Wang S, et al. Differences in the Clinical and Hematological Characteristics of COVID-19 Patients with and without Type 2 Diabetes. J Diabet Res. 2020; 2020:1038585.
[12] Bai Y, Yao L, Wei T, Tian F, Jin DY, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020; 323:1406–1407.
[13] Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, et al. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020; 106:1132–1141.
[14] Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Connolly AMF. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet. 2021; 398:599-607.
[15] Raisi-Estabragh Z, Cooper J, Salih A, Raman B, Lee AM, et al. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart. 2022; 109:119-126.
[16] Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069.
[17] Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respirat J. 2020; 55:2000547.
[18] Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Molecul Sci. 2022; 23:3636.
[19] Sarkar S, Khanna P, Singh AK. the impact of neutrophil-lymphocyte count ratio in COVID-19: a systematic review and meta-analysis. J Intens Care Med. 2022; 37:857–869.
[20] Li J, Zhang K, Zhang Y, Gu Z, Huang CX. Neutrophils in COVID-19: recent insights and advances. Virology J. 2023; 20:169.