Full Text
Introduction
Breast cancer (BC) is the most prevalent cancer worldwide. It has now surpassed lung cancer as the leading cause of global cancer incidence in 2020, with an estimated 2.3 million new cases, representing 11.7% of all cancer cases [1]. The estimated number of prevalent cases in India in 2016 was 526000 for breast cancer [2]. As per the Globocan data 2020, in India, BC accounted for 13.5% (178361) of all cancer cases and 10.6% (90408) of all deaths with a cumulative risk of 2.81.
Current trends point out that a higher proportion of the disease is occurring at a younger age in Indian women, as compared to the West [3]. Breast cancer remains the leading cause of death from malignant tumors in women in the world despite the effectiveness of initial diagnostics and the improvement of pharmacotherapy in the recent past [4].
In spite of tremendous advances, breast cancer has continued to remain an enigma. Surgery still has a central role to play in the management of breast cancer though there has been a gradual shift towards more conservative procedures and breast-conserving therapy (BCT) is becoming widely used [5]. In 1990, National Institutes of Health (NIH) released a consensus statement recommending the practice of breast conserving surgery (BCS) with adjuvant radiotherapy instead of mastectomy for the treatment of early-stage (stage I or II)breast cancer, whenever possible [6].
Breast conservation surgery (BCS) may be defined as combination of conservative surgery for resection of primary tumor with or without surgical staging of axilla, followed by radiotherapy, with or without adjuvant systemic therapy. Modified radical (Patey) mastectomy (MRM) the more commonly performed procedure, is indicated for large tumors (in relation to the size of the breast), central tumors beneath or involving the nipple, multifocal disease, local recurrence or patient preference. For women diagnosed with early- stage breast cancer, following the initial treatment, survival with breast conserving therapy (BCT) is comparable to that achieved with mastectomy with respect to disease-free survival, distant-disease-free survival, or overall survival [7]. Furthermore, a population-based cohort from the Danish Breast Cancer Cooperative Group (DBCG) concluded that patients assigned to BCS have a better survival than patients assigned to mastectomy [8].
There is a significantly better, social, emotional, and physical adjustment after BCS and in addition fewer surgical site complications with desirable cosmetic outcomes as compared to MRM [9, 10]. All these results influence the treatment decision while selecting a surgical procedure and the choice depends on a plethora of factors which includes, the stage at the time of presentation, the availability of resources and patient preference [11, 12].
Postoperative complications can demoralize patients and potentially delay adjuvant treatment, leading to adverse outcomes [12]. Due to a paucity of data in this regard from these parts of the country, there is a further need for research to explore this aspect. This study attempts to compare early postoperative morbidity, cost of surgical treatment and quality of life after surgery in patients undergoing breast conservation surgery (BCS), to that achieved with MRM in a tertiary care hospital in Thrissur, Kerala.
Materials and methodology
Patients with breast carcinoma admitted for surgical intervention in General Surgery and Onco-surgery departments of Jubilee Mission Hospital from January 2021 to July 2022 were included in this prospective cohort observational study, after obtaining approval from Institution Ethics Committee (34/21/IEC/JMMC & RI).
All patients aged between 20 and 75 years, proven to have carcinoma breast by triple assessment and admitted for surgical intervention (BCS or MRM) during the study period were included in the study. Patients with metastatic or locally advanced breast cancer, previous chest wall irradiation, involvement of the breast skin, patient refusal, patients on anticoagulants and antiplatelet therapy, recurrent breast cancer and pregnant women were excluded. A total of 119 cases which included 2 male patients and 117 female patients, admitted during the study period were evaluated. 1 patient who was not willing for the study and 12 patients on anticoagulant or antiplatelet drugs (including both male patients) were excluded.
Patients selected for BCS had small tumour size, favourable breast-tumour ratio, unifocal tumour, with no contra-indication for radiation. Patients with small breast / large tumour, multifocal tumour, central tumour, having lymph node involvement only, poorly differentiated tumour and patient preference were selected for MRM.
Patients were diagnosed through triple assessment and after clinical evaluation, underwent diagnostic, staging and fitness investigations. The patients more than 35 years of age were imaged by mammogram. Patient whose mammogram or USG was found inconclusive, underwent fine needle aspiration cytology (FNAC)/ core needle biopsy to confirm the diagnosis. In cases of no palpable lump, USG guided or mammography guided biopsy was taken. Staging was done by USG (ultrasound) of abdomen and CT scan to exclude distant metastasis. The pre-operative base-line investigations (i.e., Complete blood count, random blood sugar, serum creatinine, blood grouping with Rh-typing, chest x-ray, and ECG) were done.
On the basis of the findings, disease was staged clinically, according to AJCC classification. High risk factors, patient's age, stage of disease, family history of breast malignancy in first or second degree relatives, surgeon’s preference, fitness and consent for complete breast removal, and willingness for adjuvant radiotherapy were considered before planning surgical procedure. Histologic details considered in the further management of each patient included histologic type, grade, presence of lymph vascular emboli, pathologic stage, and ER/PR status.
Patients were given the choice of MRM and BCS based on their clinical presentation and investigation findings. They were counselled regarding the two procedures, treatment planned and were then assigned to one of the two groups i.e. those undergoing BCS and those undergoing MRM. After obtaining informed consent, patient’s demographic details, relevant history, clinical examination findings, BIRADS score and cytology reports were recorded in a data collection form and entered in MS excel spreadsheet.
Negative pressure suction drain was placed in all patients at the conclusion of the procedure. All the patients were instructed regarding elastic compression bandage application, upper limb exercises, wound and drain care. Patients discharged with drain were instructed to record the drain output daily. Drain was removed in the outpatient department once the output reduced to less than 25 ml/ day. Total number of days to drain removal was recorded. The surgical team routinely monitored all patients after intervention. The follow-up scheme consisted of breast examination, clinical examination in OPD & ultrasonography if indicated. All patients were followed up at three points in the postoperative period i.e., post-operative day 4 (POD<4) or day of discharge taken as first point, post-operative day 10 (POD 10) or day of first review, as second point and 3 months review taken as third point for purposes of data collection. Post-operative complications like seroma, lymphorroea, flap necrosis, wound infection were recorded on the above mentioned days.
The patients were followed up for three months. During this period, they were assessed for any further complications, local recurrence, distant metastases and any emotional or physical disturbances. At the end of 3 months, all patients underwent assessment of their quality of life using SF12 survey (Annexure 1) and details of adjuvant therapy if any noted. SF12 survey utilises physical and mental components for QOL assessment. Each component has 4 domains, which were evaluated with a predetermined questionnaire. The physical component score was obtained from the 4 aspects i.e., bodily pain, physical role, general health and physical functioning. The mental component score was derived from the 4 aspects i.e., mental health, emotional role, vitality and social functioning. Assessment of the quality of life and assimilation of the scores into two components were done according to instructions in standard protocol of the SF 12 analysis.
Statistical methods
Study data was tabulated in the excel spreadsheet. Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, version 20.0; IBM Corp., Armonk, NY) was used for statistical analysis. Categorical variables were represented by frequency and percentage. Numerical variables were represented by mean and standard deviation. Pearson Chi square test and binary logistic regression were performed to compare categorical variables between BCS and MRM. Independent sample t- test was performed to compare numerical variables between BCS and MRM. Multinomial logistic regression was performed to find the relationship between variables. P values less than 0.05 were considered to be statistically significant.
Results
This prospective observational study was done amongst breast carcinoma patients admitted for surgical intervention in our center. The study spanned 18 months and data from all 106 participants were available for analysis.
Out of 106 patients in the study population, the proportion of patients who underwent MRM and BCS were 75/106 (70.75%) and 31/106 (29.25%) respectively. The age at presentation in BCS group varied from 32 to 73 years with a mean age of 47 ± 9.56 years and in MRM group from 23 to 72 years with a mean age of 55 ± 11.7 years (Table1). We observed that, the highest number of cases were in the 5th and 6th decades in BCS and MRM groups respectively (p value 0.011) (Figure 1).
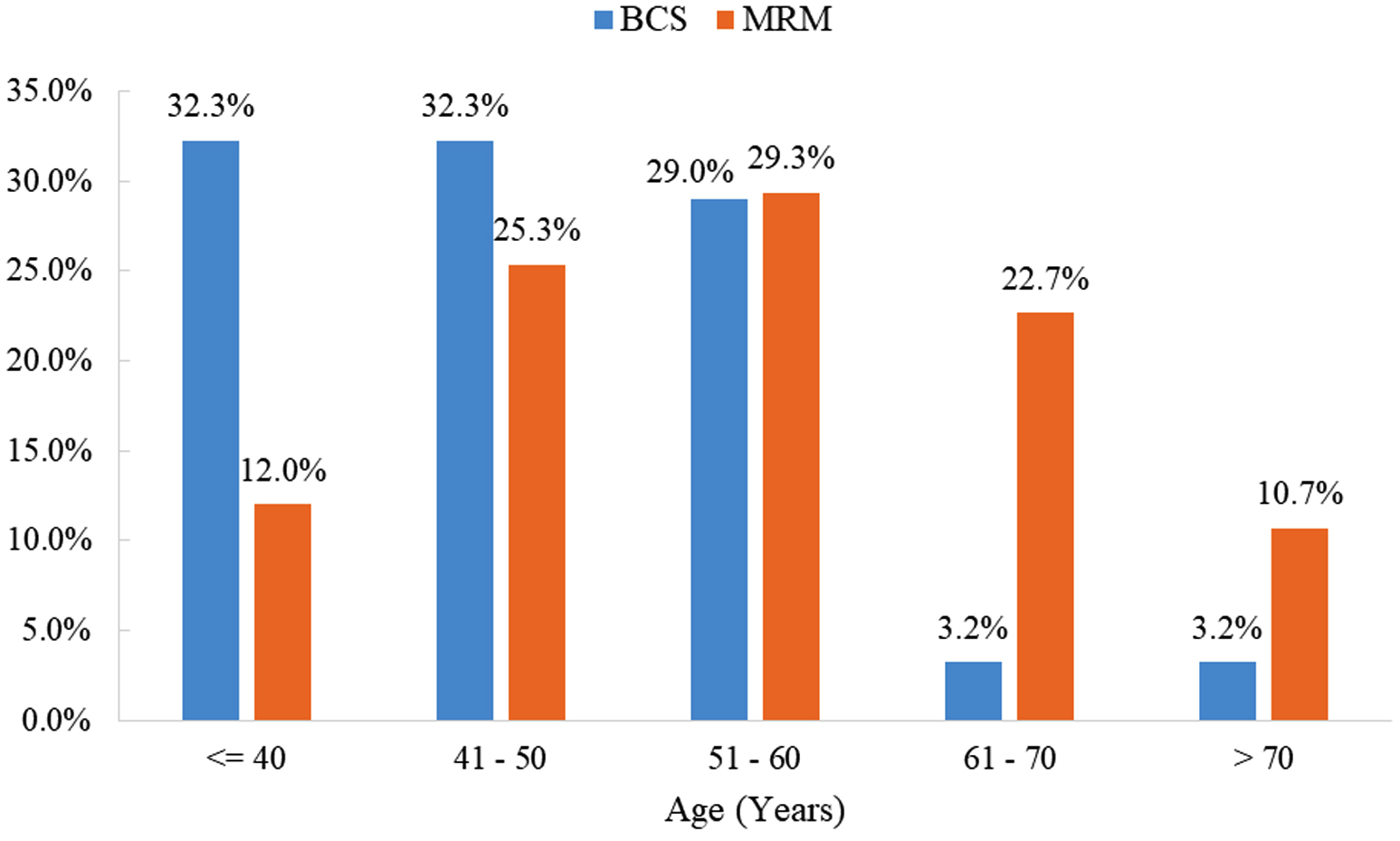
Figure 1: Distribution of patients according to age at presentation for BCS and MRM (n = 106, p value 0.011).
The study group comprised entirely of female patients (100%), as the two male patients with breast cancer during the study period were excluded due to their anticoagulant medication, Comparison of menstrual status of patients showed, 45.2% in the reproductive age group for BCS and 62.7% of patients post-menopausal in MRM group (Figure 2). This difference was not statistically significant (p value 0.091).
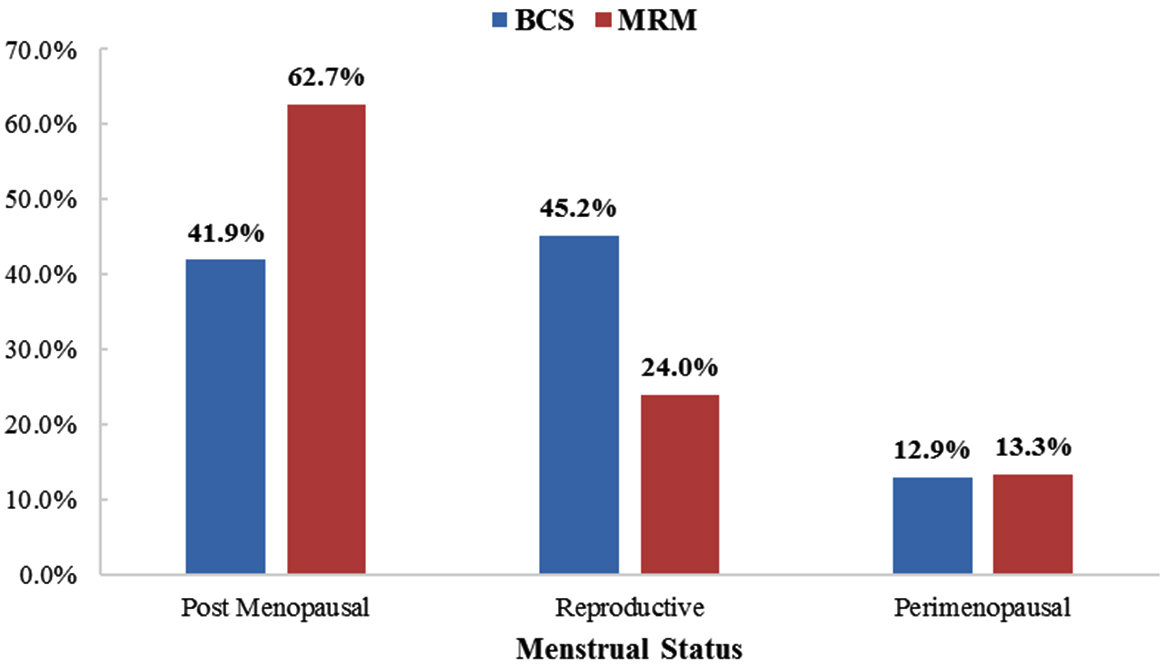
Figure 2: Comparison of menstrual status of patients at presentation for BCS and MRM (n = 106 p value 0.091).
Comparison of the BIRADs grade of radiological assessment between the two groups showed that, in the BCS group most (64.5%) patient’s where BIRADS 4 grade, whereas in the MRM group they were in both BIRADS 4 (41.3%) & BIRADS 5 (42.7%) grades. This difference in BIRADS grades between the two groups was statistically significant (p value of 0.001). We also noted a significantly higher number (8 patients) of nulliparous women in MRM group (p value 0.016) in comparison (Table 1).
Table 1: Comparison of demographic profile, clinical evaluation and radiological assessment (BIRADS Grade) of patients.
|
Demographic profile and Clinical evaluation
|
BCS (N = 31)
|
MRM (N = 75)
|
p value
|
|
Age (mean± SD)
|
47.0± 9.56
|
55.0±11.7
|
0.016**
|
| |
Post-menopausal
|
13 (41.9%)
|
47 (62.7%)
|
0.091
|
| |
Reproductive age
|
14 (45.2%)
|
18 (24.0%)
|
| |
Peri menopausal
|
4 (12.9%)
|
10 (13.3%)
|
|
Family history of carcinoma
|
4 (12.9%)
|
13 (17.3%)
|
0.565
|
|
Early menarche <13 yrs
|
5 (16.1%)
|
10 (13.3%)
|
0.665
|
|
Nulliparous
|
0 (0.0%)
|
8 (10.7%)
|
0.016**
|
|
First child birth (Years)
|
| |
< 25
|
26 (83.9%)
|
47 (62.7%)
|
0.262
|
| |
25 – 30
|
5 (16.1%)
|
19 (25.3%)
|
| |
> 30
|
0 (0.0%)
|
1 (1.3%)
|
|
Presenting complaints
|
| |
Lump
|
31 (100.0%)
|
72 (96.0%)
|
0.146
|
| |
Nipple discharge
|
1 (3.2%)
|
5 (6.7%)
|
0.463
|
| |
Pain
|
4 (12.9%)
|
5 (6.7%)
|
0.312
|
| |
Axillary swelling
|
0 (0.0%)
|
3 (4.0%)
|
0.146
|
| |
Ulcer
|
1 (3.2%)
|
5 (6.7%)
|
0.463
|
| |
Nipple retraction
|
0 (0.0%)
|
2 (2.7%)
|
0.237
|
|
Mammography, BIRADS Grade
|
| |
2
|
3 (9.7%)
|
0 (0.0%)
|
0.000**
|
| |
3
|
5 (16.1%)
|
4 (5.3%)
|
| |
4
|
20 (64.5%)
|
31 (41.3%)
|
| |
5
|
2 (6.5%)
|
32 (42.7%)
|
| |
6
|
1 (3.2%)
|
8 (10.7%)
|
** p value < 0.05 is significant.
We also observed that the number of patients in clinical Stages 3A and Stage 3B disease are significantly higher in MRM as compared to BCS (13.7% and 12.3%) vs (0.0% and 3.3%) respectively. For the comparison of clinical staging, binary logistic regression was performed and the difference in clinical staging between groups is of statistical significance (p value <0.05) (Table 2). The core biopsy HPE reports are given in Table 3.
Table 2: Clinical staging of breast cancer patients (*after excluding malignant phylloides).
|
T stage
|
Group 1 BCS
(n = 31)
|
Group 2 MRM
(n = 75)
|
Total (n = 106)
|
p value
|
|
T1
|
5 (16.1%)
|
9 (12%)
|
14 13.2%)
|
0.439
|
|
T2
|
22 (71%)
|
47 (62.7%)
|
69 65.1%)
|
|
T3
|
2 (6.5%)
|
6 (8%)
|
8 (7.5%)
|
|
T4
|
2 (6.5%)
|
13 (17.3%)
|
15 (14.2%)
|
|
N-stage N0
|
18 (58.1%)
|
28 (37.3%)
|
46 (43.4%)
|
0.054
|
|
N1
|
13 (41.9%)
|
43 (57.3%)
|
56 (52.8%)
|
|
N2
|
0 (0.0%)
|
4 (5.3%)
|
4 (3.8%)
|
|
Staging*
|
Group 1
(n = 30)
|
Group 2
(n = 73)
|
Total (n = 103)
|
|
|
Stage 1
|
0 (0.0%)
|
1 (1.4%)
|
1 (1.0%)
|
|
|
Stage 1A
|
5 (16.7%)
|
5 (6.8%)
|
10 (9.7%)
|
|
|
Stage 1B
|
0 (0.0%)
|
1 (1.4%)
|
1 (1.0%)
|
|
|
Stage 2A
|
11 (36.7%)
|
21 (28.8%)
|
32 (31.1%)
|
0.042**
|
|
Stage 2B
|
13 (43.3%)
|
26 (35.6%)
|
39 (37.9%)
|
|
|
Stage 3A
|
0 (0.0%)
|
10 (13.7%)
|
10 (9.7%)
|
|
|
Stage 3B
|
1 (3.3%)
|
9 (12.3%)
|
10 (9.7%)
|
|
** p value < 0.05 is significant
Table 3: Core biopsy HPE reports.
|
Core biopsy HPE reports
|
BCS (n = 31)
|
MRM (n = 75)
|
|
Bilateral lobular carcinoma
|
0 (0.0%)
|
1 (1.3%)
|
|
Epithelial hyperplasia with atypia
|
4 (12.8%)
|
1 (1.3%)
|
|
High grade DCIS
|
1 (3.2%)
|
0 (0.0%)
|
|
IDC
|
15 (48.3%)
|
50 (66.6%)
|
|
IDC mucinous type
|
0 (0.0%)
|
2 (2.6%)
|
|
IDC NOS
|
7 (22.5%)
|
15 (20.0%)
|
|
IDC NOS with ECIS
|
0 (0.0%)
|
1 (1.3%)
|
|
IDC with lobular carcinoma change
|
1 (3.2%)
|
0 (0.0%)
|
|
Invasive lobular carcinoma
|
0 (0.0%)
|
2 (2.6%)
|
|
Matrix producing carcinoma
|
0 (0.0%)
|
1 (1.3%)
|
|
Metastatic breast carcinoma or malignant phylloid
|
0 (0.0%)
|
1 (1.3%)
|
|
Mucinous carcinoma
|
0 (0.0%)
|
1 (1.3%)
|
Proportion of patients in our study with early post-operative complications, noted in BCS group was 9 out of 31 (26.66%) and 20 out of 75 (29%) for MRM and this incidence was comparable (p value 0.80). Incidence of individual complications in the two groups and the comparison were as follows: seroma (BCS, 16.1% vs MRM, 13.3% p value 0.710), flap necrosis (3.2% vs. 2.7% p value 0.87), wound dehiscence (3.2% vs. 5.3% p value 0.63) and infection (6.5% vs. 5.3% p value 0.823) and the average number of days of lymphorrhoea with time to drain removal (15 ± 9.76 days in BCS vs. 15.7 ± 7.49 days for MRM p value 0.220). Comparison of the two groups showed no statistically significant difference for complications (Table 4). Furthermore, patients at the 3 months follow up showed no significant difference in the occurrence of complication between the two groups. We also noted that 10 patients in the MRM group and 2 in the BCS group had persistent lymphorrhoea lymphorrhoea. Figure 3 conveys the number of days after surgery for both lymphorrhoea and the drain removal.
Table 4: Early post-operative complications and complications at 3 months.
Early post-operative complications
|
BCS (n = 31)
|
MRM (n = 75)
|
p value
|
|
Seroma
|
5 (16.1%)
|
10 (13.3%)
|
0.710
|
|
Wound infection
|
2 (6.5%)
|
4 (5.3%)
|
0.823
|
|
Wound dehiscence
|
1 (3.2%)
|
4 (5.3%)
|
0.630
|
|
Flap necrosis
|
1 (3.2%)
|
2 (2.7%)
|
0.876
|
|
Number of days of lymphorrhoea/ time to drain removal
Mean ± SD
|
15.0 ± 9.76
|
15.7 ± 7.49
|
0.220
|
|
Post-operative complications at 3month
|
|
No complication
|
28 (90.3%)
|
59 (78.7%)
|
0.322
|
|
New evidence of metastasis
|
0 (0.0%)
|
3 (4.0%)
|
|
Lymphorrhoea
|
2 (6.5%)
|
10 (13.3%)
|
|
Wound infection
|
1 (3.2%)
|
3 (4.0%)
|
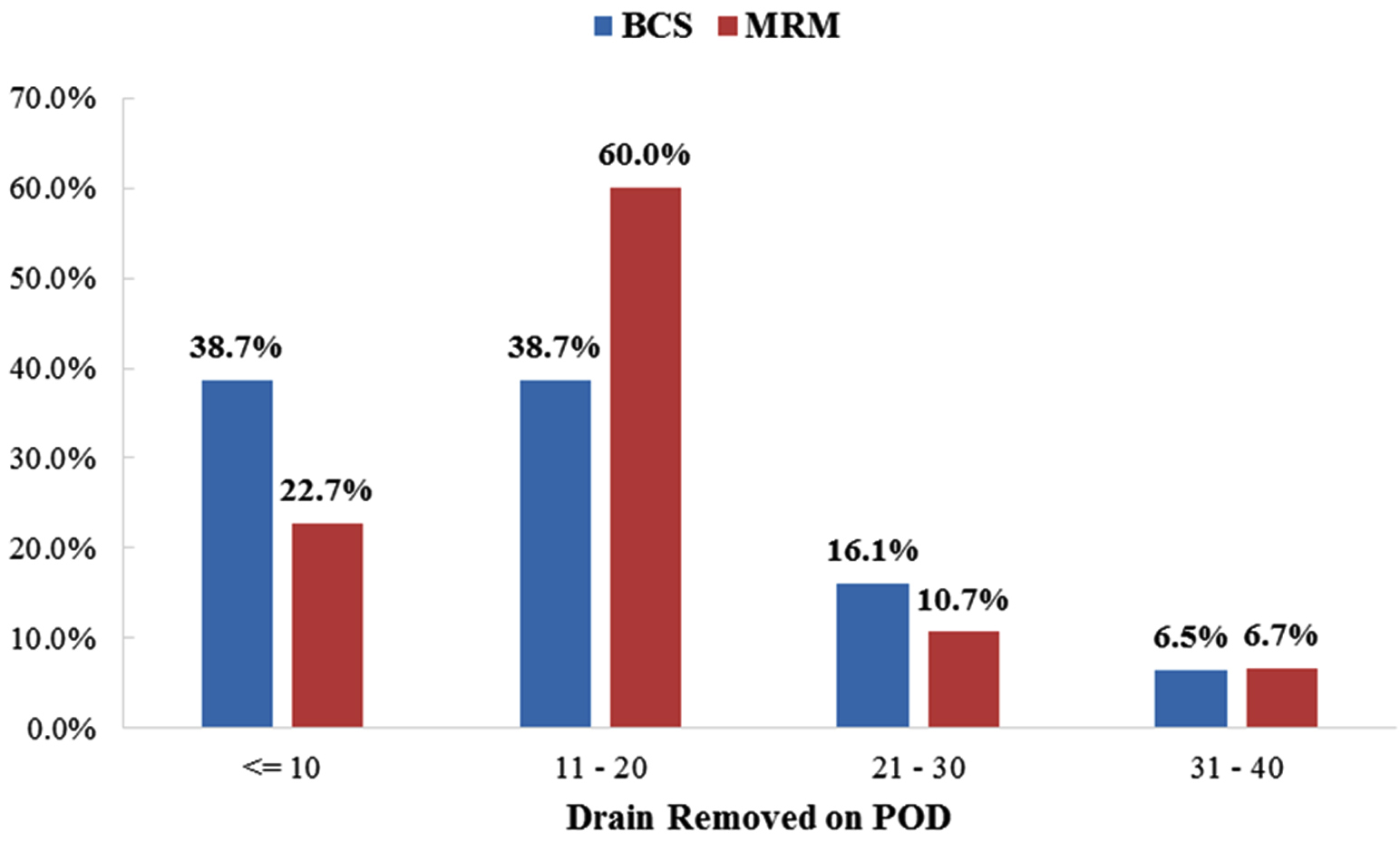
Figure 3: Lymphorrhoea and time to drain removal (in post-operative days).
The mean length of hospital stay was 3.17 ± 2.57 days for MRM and 2.48 ± 1.90 days for BCS and the difference in mean length of stay was not found to be statistically significant (p value 0.479) (Figure 4).
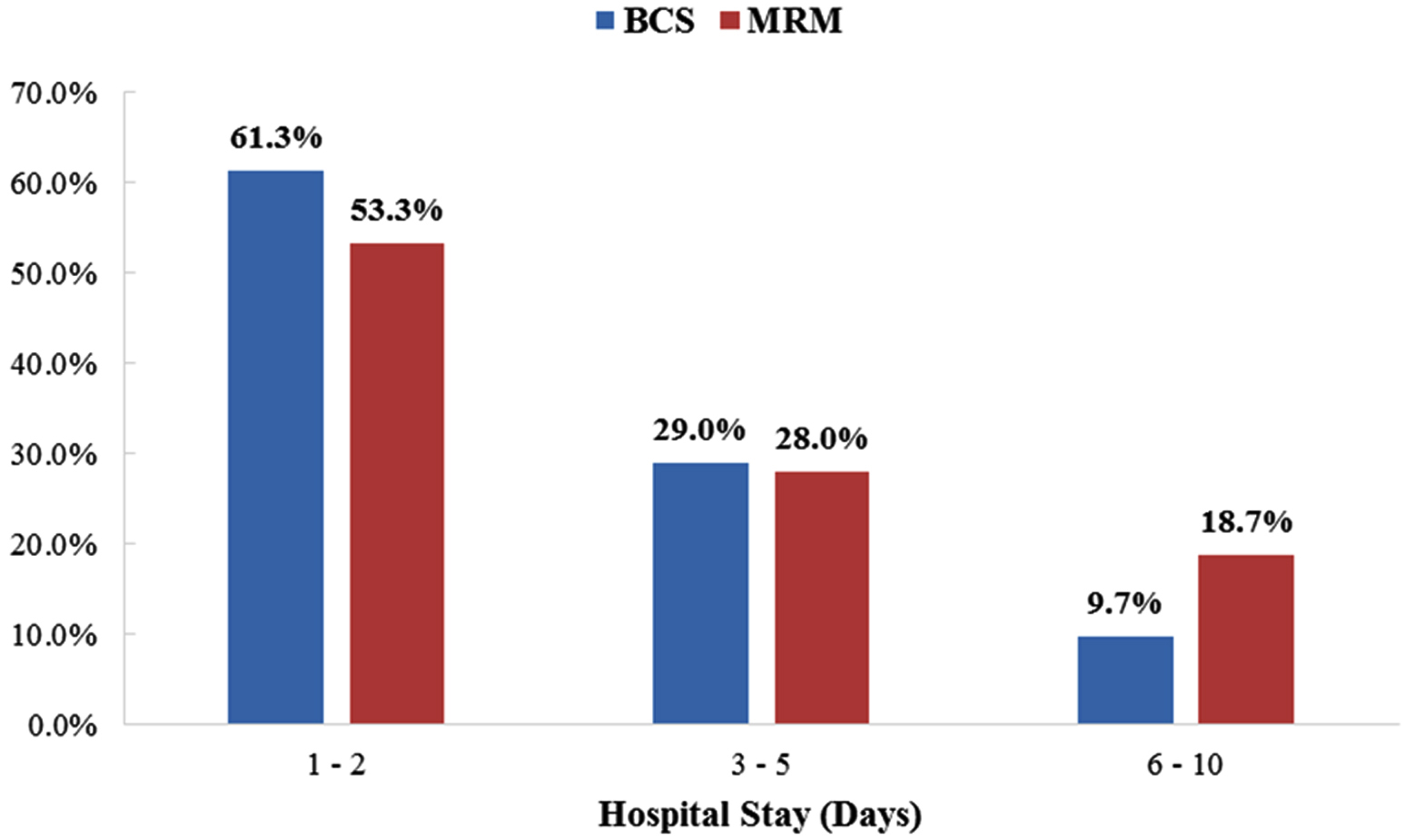
Figure 4: Duration of hospital stay (days).
Analysis of cost of treatment for MRM as compared to BCS using binary logistic regression showed statistically significant higher cost for MRM (p value 0.041) (Table 5).
Table 5: Cost of treatment.
|
Cost of treatment (Rs)
|
BCS (n = 31)
|
MRM (n = 75)
|
p value
|
|
< 25,000
|
5 (16.1%)
|
3 (4.0%)
|
0.041**
|
|
25,000 - 50,000
|
23 (74.2%)
|
54 (72.0%)
|
|
50,000 - 1,00,000
|
3 (9.7%)
|
18 (24.0%)
|
** p value < 0.05 is significant
Overall score for Q O L as assessed by SF 12, showed no significant difference between the groups. However, a comparatively poor mental health component score was observed in the BCS group which was statistically significant (mean 8.9 ± 1.832 in BCS vs 8.12 ± 1.85 MRM p value 0.029) (Table 6).
Table 6: Assessment by SF12 QOL survey.
|
SF12 QOL survey/ group
|
Number of cases
|
Mean score
|
SD
|
p value
|
|
PCS
|
| |
BCS
|
31
|
4.968
|
±1.080
|
0.876
|
| |
MRM
|
75
|
5.013
|
±1.466
|
|
MCS
|
| |
BCS
|
31
|
8.903
|
±1.832
|
0.029**
|
| |
MRM
|
75
|
8.120
|
±1.585
|
|
SF12
|
| |
BCS
|
31
|
13.87
|
±2.306
|
0.148
|
| |
MRM
|
75
|
13.13
|
±2.396
|
** p value< 0.05 is significant
HPE pattern among the groups, showed the most common type was IDC NOS type (71% & 84% in BCS & MRM respectively). Other histological types of IDC noted include mucinous, papillary & tubular, 2 cases of ILC in BCS and 1 in MRM for a total of 3 cases. The lympho-vascular invasion was seen to be higher in MRM group (40%) when compared with BCS group (19.4%) and this difference was statistically significant p value 0.035 (Table 7).
Table 7: Histopathological examination findings.
|
Histopathological examination
|
BCS (N = 31)
|
MRM (N = 75)
|
p value
|
|
IDC mucinous
|
0 (0.0%)
|
2 (2.7%)
|
0.103
|
|
IDC NOS
|
23 (74.2%)
|
63 (84.0%)
|
|
IDC NOS+ Mucinous
|
0 (0.0%)
|
1 (1.3%)
|
|
IDC NOS+ ILC NOS
|
0 (0.0%)
|
1 (1.3%)
|
|
IDC NOS+ Malignant phylloid
|
2 (6.5%)
|
1 (1.3%)
|
|
IDC NOS+ Papillary carcinoma
|
1 (3.2%)
|
1 (1.3%)
|
|
IDC Papillary carcinoma
|
1 (3.2%)
|
1 (1.3%)
|
|
IDC Tubular
|
1 (3.2%)
|
0 (0.0%)
|
|
ILC NOS
|
2 (6.5%)
|
5 (6.7%)
|
|
Malignant phylloides
|
1 (3.2%)
|
0 (0.0%)
|
|
Lymph node status
All node negative
|
14 (45.2%)
|
35 (46.7%)
|
0.644
|
|
Grade
|
|
1
|
6 (19.4%)
|
8 (10.7%)
|
0.388
|
|
2
|
10 (32.3%)
|
29 (38.7%)
|
|
3
|
12 (38.7%)
|
38 (50.7%)
|
|
DCIS
|
16 (51.6%)
|
33 (44.0%)
|
0.475
|
|
Paget’s disease
|
0 (0.0%)
|
4 (5.3%)
|
0.097
|
|
Lympho-vascular invasion
|
6 (19.4%)
|
30 (40.0%)
|
0.035**
|
|
IHC
|
|
ER
|
20 (64.5%)
|
44 (58.7%)
|
0.445
|
|
PR
|
18 (58.1%)
|
38 (50.7%)
|
0.385
|
|
HER2NEU
|
10 (32.3%)
|
28 (37.3%)
|
0.699
|
|
IHC type
|
|
A
|
14 (45.2%)
|
27 (36.0%)
|
0.598
|
|
B
|
6 (19.4%)
|
21 (28.0%)
|
|
Basal
|
6 (19.4%)
|
20 (26.7%)
|
|
HER
|
4 (12.9%)
|
7 (9.3%)
|
** p value< 0.05 is significant
Post operatively, 2 patients in BCS group diagnosed to have malignant phylloides tumour were advised follow up and the remaining advised adjuvant therapy as shown (Table 8).
Table 8: Adjuvant therapy.
|
Adjuvant therapy
|
BCS (N = 31)
|
MRM (N = 75)
|
p value
|
|
Adjuvant RT
|
20 (64.5%)
|
35 (46.7%)
|
0.107
|
|
Adjuvant CT
|
25 (80.6%)
|
66 (88.0%)
|
0.335
|
|
Adjuvant HT
|
17 (54.8%)
|
45 (60.0%)
|
0.624
|
Discussion
Recent advancements in breast cancer surgery have focused on three key areas: patient recovery, oncological safety and the best possible cosmetic result. Most patients undergoing breast conservation therapy need adhere to a therapeutic regimen that encompasses a combination of chemotherapy and radiotherapy. A stringent adherence to such regimens can portend favorable surgical outcomes. However, a delay in treatment due to any comorbidity and post-operative complication can have an adverse impact on the patients’ overall survival [12]. The concept that lumpectomy and breast irradiation could adequately control local extent of the disease did not receive widespread acceptance until several decades later although breast conservation was proposed as far back as the 1930s. Seven prospectively randomized studies involving thousands of patients with follow-up periods of more than 2 decades have demonstrated that local tumor control and disease-free survival (DFS) in BCT are comparable to that with radical mastectomy. Hence, BCT is becoming a widely used therapy for breast cancer [7, 13].
The present study was done among patients with breast carcinoma admitted for surgical intervention and the purpose was to compare early postoperative outcomes of patients undergoing breast conservation surgery BCS to that achieved with MRM.
We observed that, a higher proportion (70.75%) of our study patients chose to undergo MRM rather than BCS. Our patients who preferred MRM, chose this option due to lack of radiotherapy in our centre and their perception of the slightly higher risk of disease recurrence. Our results were similar to the research findings of Kadam SS et al who showed that 79.1% females opted for MRM and 20.9% females opted for BCS [14]. Lower rates of BCT seen in developing countries has been attributed to a lack of awareness among patients, limited accessibility to adjuvant therapy, patient's preference to avoid multiple hospital visits, presentation at advanced stage and fear of complication of radiotherapy and disease recurrence [15].
In our study, the mean age at presentation for BCS was 47 years and for MRM 55 years. We observed, in BCS group, a relatively younger population (20 out of 31 cases < 50 years) as compared to MRM group (58 out of 75 cases aged between 40 and 70 years). 45.2% of patients in the BCS group were of the reproductive age, while the highest number of patients were post-menopausal (62.7%) in MRM group. Our data compares well with the study by Teh et al who reported that, patients older than 60 years were significantly more likely to receive mastectomy rather than BCS as the initial treatment, as majority of patients were concerned about the oncological outcome, regardless of the type of surgery they underwent [16]. Our study population was comprised entirely of females, as the two male patients got excluded since they were on anti-coagulant / antiplatelet drugs. Overall global incidence of male breast cancer is 1%.
We observed that, a higher proportion of patients had T2 stage tumour in both BCS and MRM groups {22 (71%) & 47 (62.7%) respectively}. We also noted that, more patients with N0 stage {18(58.1%)} underwent BCS and while more patients with N1 stage {43(57.3%)} underwent MRM. In addition, we observed patients with a higher BIRADS score (5 & 6) underwent MRM rather than BCS. Patients with high risk factors like nulliparity, family history of carcinoma, hormone therapy were also seen in a higher proportion in the MRM group. This we attribute to the understanding that, while counseling on surgical options, the surgeon has to take into account the tumor characteristics (size, site, nodal status, systemic risk) and patient characteristics (breast volume). Joty et al concluded in their findings that BCS offers less trauma, infection and hospital stay; better aesthetic outcome and quality of life than MRM, making it more deserving of being promoted clinically in the treatment of early-stage breast cancer [17].
Among the early post-operative complication which were studied, the most common postoperative complication noted was that of seroma in 16.1% of cases for BCS and 13.3% in MRM followed by wound infection and dehiscence seen in 6.5% for BCS and 5.3% in MRM. We also noted that, lymphorrhoea persisted for 15.0 ± 9.76 and 15.7 ± 7.49 days in BCS and MRM groups respectively. Rizvi et al showed there was no significant difference in postoperative complication between MRM and BCS with regard to incidence of seroma, wound infection and dehiscence, flap necrosis and recurrence [12]. Al-Ghazal et al reported that BCS has the advantage of fewer surgical site complications with the addition of desirable cosmetic outcomes as compared to MRM [10]. Liu and Luo in their research, observed an overall lower complication rate in BCS as compared to MRM with seroma / effusion being the most common among them [18]. The incidence of seroma in the present study is comparable with that reported by Sreelesh, Oommen A [19], and Kadam et al [14].
All patients were followed up for a 3 month period with regard to occurrence of complications. We noted that 2 cases in BCS and 10 cases in MRM groups had persistent seroma or lymphorrhoea requiring aspiration and drainage. We also observed the presence of wound infection in 1 case (3.2%) of BCS group and 3 cases (4.0%) in MRM group. In addition new evidence of metastasis was noted in 3 patient post MRM (4.0%) emphasizing the need for strict adherence to treatment protocol and follow up.
The policy of early discharge of our patients with drain in situ as followed in our institute, facilitated a reduction in the hospital stay to an average of 2.48 ± 1.90 days for BCS and 3.17 ± 2.57 days for MRM groups respectively. Drain tubes were removed during outpatient review visit with a mean period of 15.0 days for BCS and 15.7 days in MRM groups. Our findings may help patients and surgeons in our country decide whether BCT or MRM is the better option in a given case and advise eligible patients that BCS is an equivalent option for those who are willing.
The cost of treatment in this study was limited to the surgical treatment alone as radiotherapy was not available in our institution. According to our study the cost of surgical treatment was significantly higher in MRM group when compared to BCS (p value 0.041). This was due to the higher procedural charge for MRM and the higher category of hospital accommodation (room) chosen by many MRM patients. Furthermore, the entire BCS procedure was performed by the operating surgeon and did not involve a plastic surgical consultant for the reconstruction.
At 3 month follow up, a health survey questionnaire was completed and recorded. SF12 QoL analysis utilize 8 domains for assessment and the reports are comparable to SF36 QoL surveys. The two components include the physical component score (bodily pain, role -physical, general health and physical functioning) and mental component score (mental health, role- emotional, vitality and social functioning). We observed that, the aggregate score of physical and mental components according to SF12 questionnaire analysis for quality of life, showed no significant difference between the two groups with regard to the overall quality of life post MRM and BCS. However, SF12 questionnaire analysis showed a higher mental component score for BCS, suggesting poorer mental health for BCS post operatively. Poorer mental health score among our patients was attributed to the uncertainty and restrictions in place during pandemic period, unavailability of radiation therapy in our center, the fear of radiation therapy complications and that of local recurrence and metastases. Our findings were similar to that of Deepa K V et al who concluded in their study that quality of life in postoperative health analysis were similar for MRM and BCS groups [20]. Freita-Silva et al also reported that there were no differences between the two surgical techniques with respect to quality of life or satisfaction with surgery [21]. However, Elmas et al, and El-Maghawry et al., in their research, reported better quality of life after BCS [22, 23].
Limitations of the study: The small sample size owing to the SARS Cov 2 virus pandemic during the study period and higher number of patients on antiplatelet post SARS Cov2 infection highlighted the need for larger randomized trial, in the post COVID period. This was a single-center prospective study and the findings cannot be generalized as information was limited to that which was collected during hospital admissions for surgical treatment and the QoL was assessed based on a questionnaire survey after the surgery. The small sample size and short follow-up period in our study limits the comparison of BCT and MRM, and we hope that this might be addressed by our future studies.
Conclusion
Our data shows that a higher percentage of our patients underwent modified radical mastectomies. The study is significant from our perspective, as it reveals the fact that most of the patients in these parts, present slightly later in the disease and prefer modified radical mastectomy. In our center, overall early postoperative morbidity rates for both BCS and MRM are low and comparable. Seroma and lymphorrhoea were the most common early postoperative complications observed and this equally affected both groups of patients. Based on the results we obtained, overall QoL was seen to be equitably affected in BCS and MRM group of patients postoperatively. Eligible patients with breast cancer should be educated regarding breast conservative and oncoplastic procedures and the same deserves to be promoted. The practice of early discharge and continued domiciliary patient care has positive influence on patient recovery and wellbeing. Further comprehensive studies and prospective matched trials are required to better assess and compare the impact of economic burden on this population.
Acknowledgement
Departments of General surgery, Oncosurgery, and Research department, Jubilee Mission Medical College and Research Institute.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.
[2] India State-Level Disease Burden Initiative Cancer Collaborators. The burden of cancers and their variations across the states of India: the global burden of disease study 1990-2016. Lancet Oncol. 2018; 19:1289–1306.
[3] Mehrotra R, Yadav K. Breast cancer in India: Present scenario and the challenges ahead. World J Clin Oncol. 2022; 13:209–218.
[4] Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005; 293:1245–1256.
[5] Wang L, Ouyang T, Wang T, Xie Y, Fan Z, et al. Breast-conserving therapy and modified radical mastectomy for primary breast carcinoma: a matched comparative study. Chin J Cancer Res. 2015; 27:545–52.
[6] Du X, Freeman DH, Syblik DA. What drove changes in the use of breast conserving surgery since the early 1980s? The role of the clinical trial, celebrity action and an NIH consensus statement. Breast Cancer Res Treat. 2000; 62:71–79.
[7] Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
[8] Christiansen P, Carstensen SL, Ejlertsen B, Kroman N, Offersen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival—a population based study by the Danish Breast Cancer Cooperative Group (DBCG), Acta Oncologica. 2018; 57:19–25.
[9] Patil BJ, Dikle SM. Role of breast conservation surgery (BCS) and modified radical mastectomy (MRM) in early breast cancer – a comparative study; MedPulse Int J Surgery. 2019; 11:1.
[10] Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer. 2000; 36:1938–1943.
[11] Marissa C, Munck L, Bock GH, Jobsen JJ, Dalen T, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016; 17:1158–1170.
[12] Rizvi FH, Khan M, Almas T, Ullah M, Shafi A, et al. Early postoperative outcomes of breast cancer surgery in a developing country. Cureus. 2020; 12:e9941.
[13] Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002; 347:567–575.
[14] Kadam SS, Tripathi P, Jagtap R, Kapoor R, Kadam T, et al. Modified radical mastectomy vs. breast-conserving surgery: current clinical practice in women with early stage breast cancer at a corporate tertiary cancer center in India. Indian J Surg Oncol. 2022; 13:322–328.
[15] Kantor O, Pesce C, Kopkash K, Barrera E, Winchester DJ, et al. Impact of the society of surgical oncology-American society for radiation oncology margin guidelines on breast-conserving surgery and mastectomy trends. J Am Coll Surg. 2019; 229:104–114.
[16] Teh YC, Shaari NE, Taib NA, Ng CH, See MH, et al. Determinants of choice of surgery in Asian patients with early breast cancer in a middle income country. Asian Pac J Cancer Prev. 2014; 15:3163–3167.
[17] Joty SM, Saiyara N, Shishir MTA, Islam F, Khan MR, et al. Comparative study between breast conservative surgery and modified radical mastectomy in early stage of breast carcinoma in a tertiary care hospital. Int J Res Med Sci. 2023; 11:794–800.
[18] Heng LIU, Chengyu LUO. Effect of breast-conserving surgery and modified radical mastectomy on quality of life of early breast cancer patients. Food Sci Technol. 2022; 42:e47021.
[19] Sreelesh LS, Oommen A. A comparative study of breast conservative surgery and modifiedradical mastectomy in early breast cancer. J. Evid. Based Med Healthc. 2016; 3:4760–4765.
[20] Deepa KV, Gadgil A, Löfgren J, Mehare S, Bhandarkar P, et al. Is quality of life after mastectomy comparable to that after breast conservation surgery? A 5-year follow up study from Mumbai, India. Qual Life Res. 2020; 29:683–692.
[21] Freitas-Silva R, Conde DM, de Freitas-Júnior R, Martinez EZ. Comparison of quality of life, satisfaction with surgery and shoulder-arm morbidity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics (Sao Paulo). 2010; 65:781–787.
[22] Elmas Ö, Çakmak GK, Bakkal BH. A comparison between modified radical mastectomy and breast-conserving surgery concerning the quality of life in patients with breast cancer under 50 years of age. Med J West Black Sea. 2021; 5:63–67.
[23] El-Maghawry HL, Amin MF, Khairy MM, Arafa AS, Nofal HA, et al. Breast-conserving therapy versus modified radical mastectomy in the early breast cancer management: oncological outcome and quality of life. Med J Cairo Univ. 2019; 87:1639–1647.