Full Text
Introduction
Human immunodeficiency virus (HIV) infection is a global pandemic with 39 million people living worldwide [1]. India too has a concentrated HIV epidemic with substantial geographical variation. As per the latest HIV estimates report (2019) of the Government, India is estimated to have around 23.49 lakh people living with HIV/AIDS (PLHIV) in 2019. The HIV epidemic has an overall decreasing trend in the country with estimated annual New HIV infections declining by 37% between 2010 and 2019 [2]. This decreasing trend is due to availability of highly active anti-retroviral therapy in India; under the guidance of National Aids Control Organization. Despite the benefits, antiretroviral therapy might cause adverse reactions which may be the main reason for drug discontinuation, switching and non-adherence. As a result, intended benefits from antiretroviral drug therapy may not be achieved or treatment failure may occur [3-5].
Antiretroviral therapy comprising of two nucleoside reverse transcriptase inhibitors (NRTIs) as a backbone and one non-nucleoside reverse transcriptase inhibitor (NNRTI) is maintained as the preferred first-line treatment regimen in adults because of its high viral suppression capacity [6]. Anti-retroviral therapy has myriads of adverse effect affecting various system of body. Efavirenz (EFV), one of the NNRTIs, has high antiretroviral efficacy and prolonged half-life allowing once daily dosing [7]. However, it crosses the blood–brain barrier (BBB) and can cause central nervous system (CNS) adverse effects [8]. As compared with structurally similar agents such as nevirapine, neuropsychiatric effects from EFV occur more frequently and with greater severity [9]. CNS adverse effects reduce the patient’s quality of life which leads to loss of confidence in the health care and the drug, which in turn makes the patient non-adherent to the medication. Scourfield et al reported that of 89 patients who switched from an EFV based ART regimen, 71% was due to CNS toxicity [10].
Most often adverse drug reactions go unnoticed or are not reported. There is a lack of awareness and inadequate training about drug safety monitoring procedures among healthcare professionals in India. Anti-Retroviral therapy induced adverse drug reactions (ADRs) may significantly impact a patient's quality of life. Increase in use of antiretroviral drugs increases the risk for ADRs, resulting in humanistic and economic burden to the HIV infected patients as well as to the society.
Concerning central nervous system related adverse drug reactions pertaining to anti-retroviral therapy in HIV positive patients will help physicians to gain a working knowledge of these adverse effects and would be beneficial to the HIV infected patients, with the ultimate goal of improving the prescription habits and improving the tolerability and effectiveness of HIV treatment by promoting the early recognition of potentially serious adverse effects.
This study was conducted to assess the pattern of central nervous system related ADRs in HIV infected patients receiving anti-retroviral therapy.
Materials and methods
A prospective observational study was conducted from May 2020 to Aug 2021 at anti-retroviral therapy center under department of Internal Medicine, GSVM Medical College, Kanpur in association with department of Pharmacology of tertiary care teaching hospitals in Uttar Pradesh, India.
Inclusion criteria: Both newly and previously registered HIV positive patients of both genders, willing to give informed written consent ,who were on anti-retroviral therapy and experienced ADRs, were included in this study.
Exclusion criteria: Patients unable to respond to verbal questions or pregnant/ lactating females or patients with concomitant disorders such as diabetes mellitus and hypertension, were excluded from this study.
Before enrolling the patients into the study, written informed consent was obtained. After enrolment baseline laboratory investigations such as hemoglobin estimation, total leukocyte count, differential leukocyte count, serum creatinine, blood urea, serum bilirubin, SGOT, SGPT, blood sugar, CD4 count etc were done. ADR monitoring was done in a systematic manner adopting both spontaneous and intensive monitoring approaches. Adverse drug reaction reporting form provided by Indian Pharmacopoeia Commission (IPC) Ghaziabad was used for data collection keeping all the norms of confidentiality.
Treatment was initiated as per national guideline in India, according to which fixed dose combination of two NRTIs (zidovudine/tenofovir + lamivudine) and one NNRTI (nevirapine/efavirenz) is recommended [11].
Each reported case of dermatological ADR was assessed for its causality by using Naranjo's probability scale [12]. Preventability was assessed using Schumock and Thornton preventability criteria [13], and severity was assessed using the modified Hartwig and Siegel scale [14]. Predictability assessment was done based on modified guidelines developed by the Council for International Organizations of Medical Sciences [15].
Statistical analysis
For the analysis of data statistically whether the observations are statistically significant or not various statistical parameters like mean, standard deviation were used. For assessing the various risk factors for the development of ADRs, the mean value of the risk factors were compared between the two groups by using t- test. To see the association between the two variables chi-square test was also used. Calculation of mean and standard deviation was done by using Microsoft Excel 2010. For applying t- test and chi-square test we used graph-pad software. In testing the statistical significance between the two means, the level of significance alpha =0.05 was used.
Results
A total of 250 patients encountered various types of ADRs during the study period. Total number of ADRs recorded was 452. Out of total ADRs (n=452) recorded, 20.35% (n=92) were ADR related to the central nervous system (Figure 1).
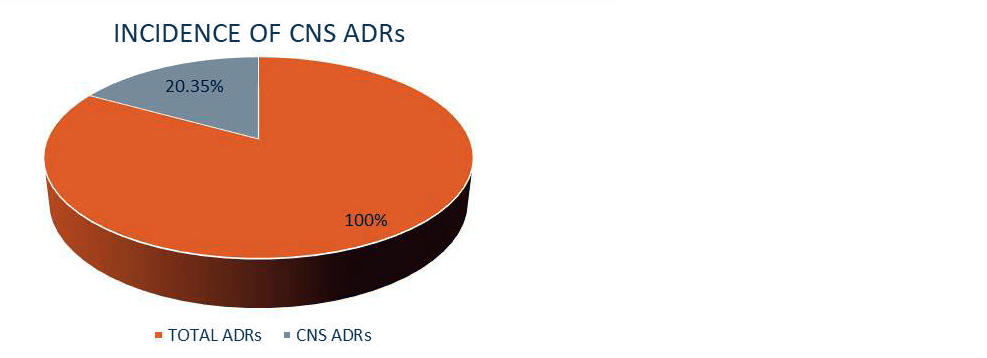
Figure 1: Incidence of CNS ADRs.
Distribution of central nervous system related ADRs: In our study among central nervous system related ADRs due to anti-retroviral therapy most common ADR found was dizziness followed by headache, insomnia, somnolence and various other ADRs (Figure 2).
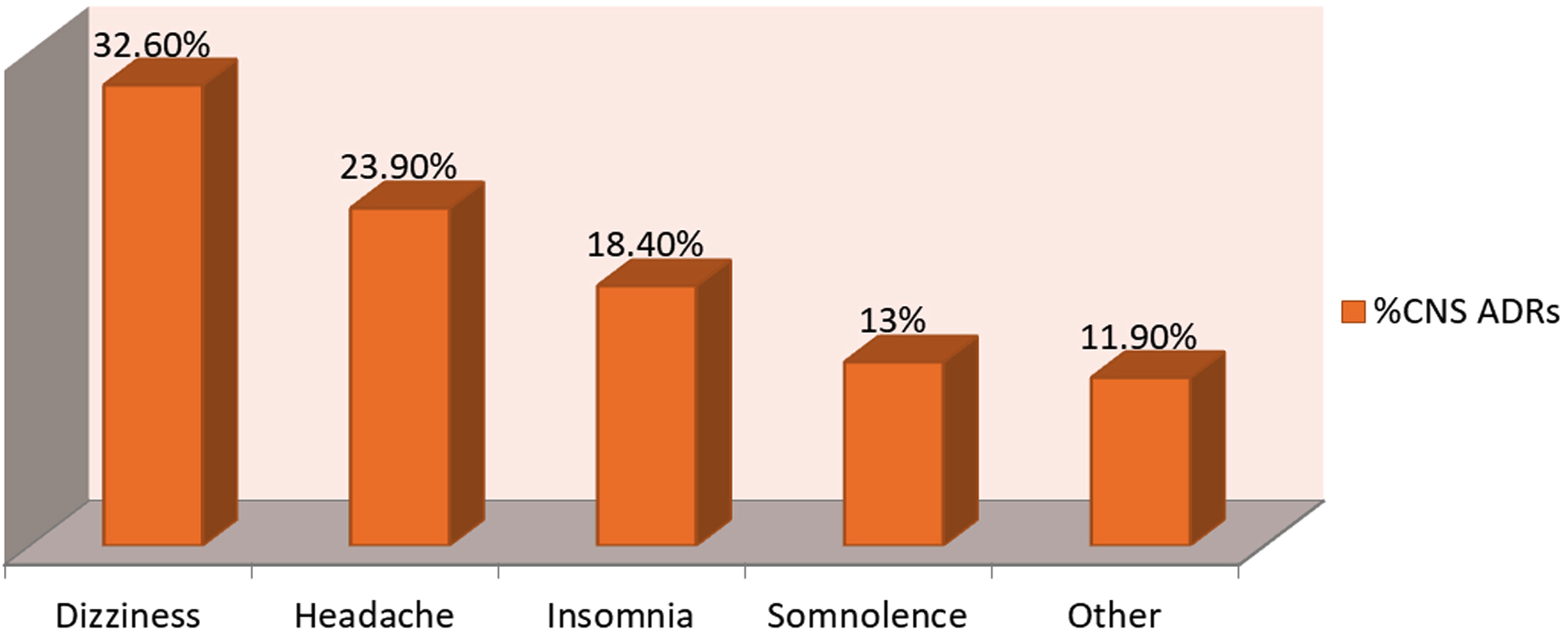
Figure 2: Distribution of CNS ADRs.
Frequency of central nervous system related ADRs due to various anti-retroviral drug regimens: In our study maximum burden of central nervous system ADRs was found due to regimen ZLE followed by ZLN, TLE and TLN. In our study there was no central nervous system related ADR encountered due to ZLAT/r regimen (Figure 3).
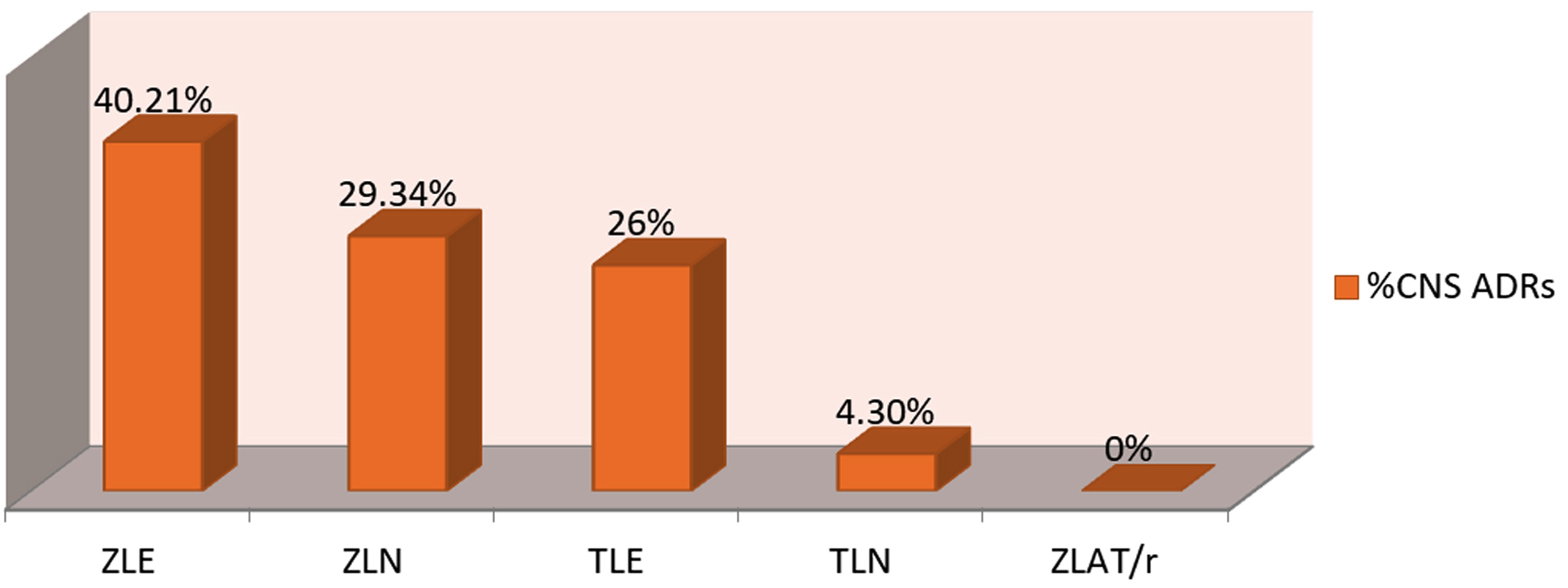
Figure 3: CNS ADRs due to different anti-retroviral regimens.
Analysis of various risk factors for development of CNS ADRs due to anti-retroviral therapy: Various demographic parameters were analyzed for the risk of developing central nervous system toxicity in the patients on anti-retroviral therapy.
By observing the table; we can make an inference that there was no statistically significant difference in gender between the two groups. However statistically significant differences were found in age, weight, CD4 cells count and anti-retroviral regimen in between the two groups.
In the group who encountered CNS related ADRs; mean age 38.28 years was higher than the mean age 34.74 in the group with no CNS related ADR and it was found statistically very significant (p<0.05, 95%CI). Age between 21-40 years was observed as a risk factor for the development of CNS toxicity and it was statistically significant (p<0.05, 95% CI).
Mean weight (51.30 kg) of patients in the group with CNS toxicity was significantly higher than the mean weight (48.85 kg) of group who did not develop CNS toxicity. Maximum burden of CNS related ADRs was observed in patients with CD4 cells count <250 cells/mm3 and it was statistically significant (p<0.05, 95% CI). The Efavirenz based regimen was responsible for the maximum burden of CNS toxicity and it was found statistically very significant (p<0.05, 95% CI). Thus; to sum up the age between 21-40 years, higher range of weight, CD4 count <250 and efavirenz based regimen were observed as a risk factor for developing central nervous system toxicity due to anti-retroviral drugs. The details of analysis are shown in the following (Table 1).
Table 1: Risk factors analysis.
|
Characteristics
|
CNS ADRs
|
|
Yes (n=69)
|
No (n=181)
|
Test result
|
α=0.05
|
|
Mean
|
SD
|
Mean
|
SD
|
p
|
|
Male
|
36
|
|
73
|
|
χ2=3.35
|
0.06
|
|
Female
|
33
|
|
108
|
|
|
Age(yr.)
|
38.28
|
10.49
|
34.74
|
8.80
|
t=2.48
|
0.007**
|
|
<20
|
16.75 (n=4)
|
2.62
|
13 (n=4)
|
4.24
|
t=1.50
|
0.18
|
|
21-40
|
32.89 (n=37)
|
4.10
|
30.67 (n=135)
|
5.36
|
t=2.70
|
0.02*
|
|
41-60
|
47.5 (n=26)
|
5.09
|
46.02 (n=41)
|
3.98
|
t=0.30
|
0.18
|
|
>60
|
61.5 (n=2)
|
0.70
|
n =1
|
|
|
|
|
Weight (kg.)
|
51.30
|
9.56
|
48.85
|
8.23
|
t=1.87
|
0.04*
|
|
<35
|
31.75 (n=4)
|
3.30
|
32 (n=9)
|
3.84
|
t=0.03
|
0.9
|
|
36-55
|
47.79 (n=44)
|
4.73
|
46.98 (n=138)
|
5
|
t=0.97
|
0.34
|
|
56-75
|
61.7 (n=20)
|
4.49
|
60.76 (n=34)
|
4.53
|
t=0.16
|
0.46
|
|
>75
|
n =1
|
|
n =0
|
|
|
|
|
CD4 Count (cells/mm3)
|
274.01
|
68.46
|
269.01
|
76.49
|
t=0.49
|
0.63
|
|
<250
|
215.5 (n=34)
|
21.85
|
223.63 (n=109)
|
16.32
|
t=1.99
|
0.02*
|
|
>250
|
330.85 (n=35)
|
46.49
|
343.19 (n=72)
|
69.44
|
t=1.08
|
0.34
|
|
Regimen
|
No. of
|
patients
|
No. of
|
patients
|
|
|
|
Efavirenz based
|
42
|
|
43
|
|
χ2=30.40
|
0.00001**
|
|
Nevirapine based
|
27
|
|
133
|
|
|
ZLAT/r (second line)
|
0
|
|
5
|
|
Distribution of ADRs according to ADR types: All reported ADRs were categorized into six types according to expanded Rawlins & Thompson classification. Majority of central nervous system related ADRs were of type-A in our study.
Causality assessment of ADRs: All ADRs were analyzed for the causality according to the Naranjo probability scale. Out of 92 CNS ADRs, the majority were in the possible category followed by the probable category. There was no ADR which was classified as doubtful or definite category (Figure 4).
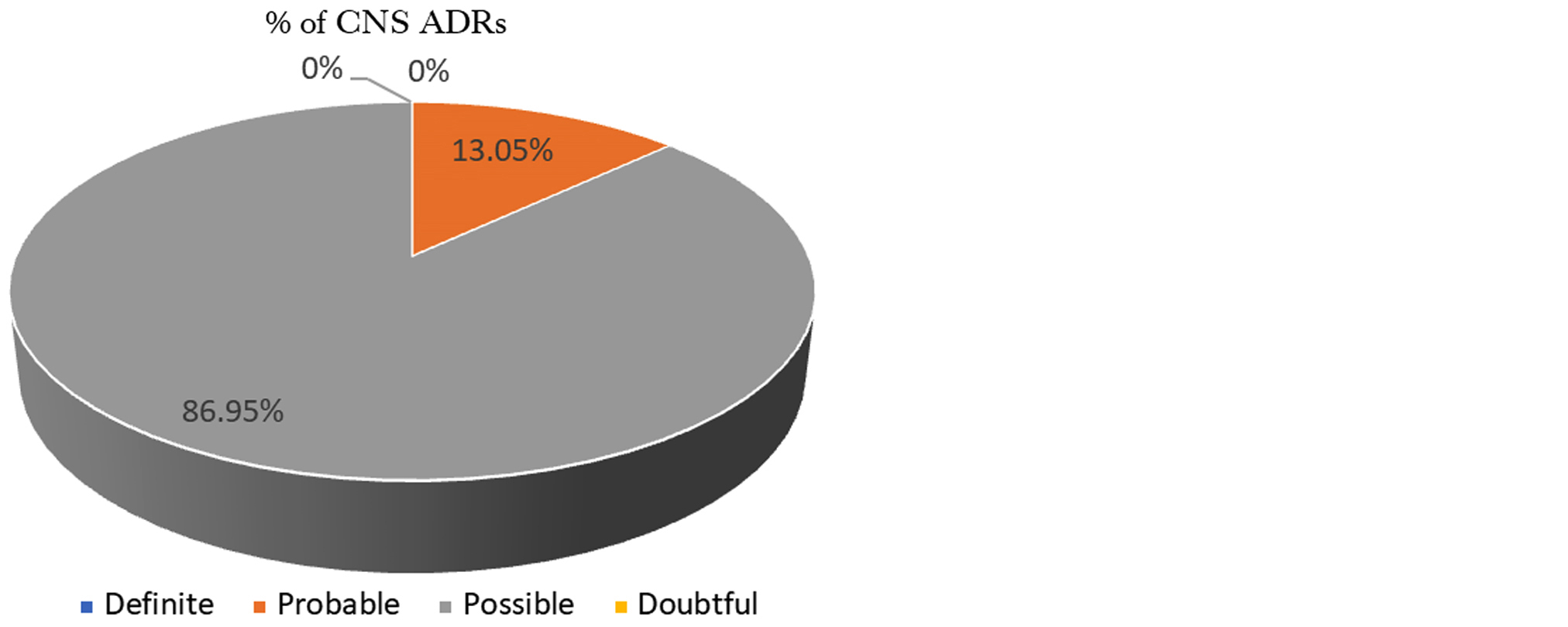
Figure 4: Causality assessment (Naranjo’s scale).
Severity assessment of ADRs: All reported central system related ADRs were analyzed for severity according to modified Hartwig severity scale. Out of the total reported ADRs, the majority were of moderate type followed by mild. No severe ADR was found (Figure 5).
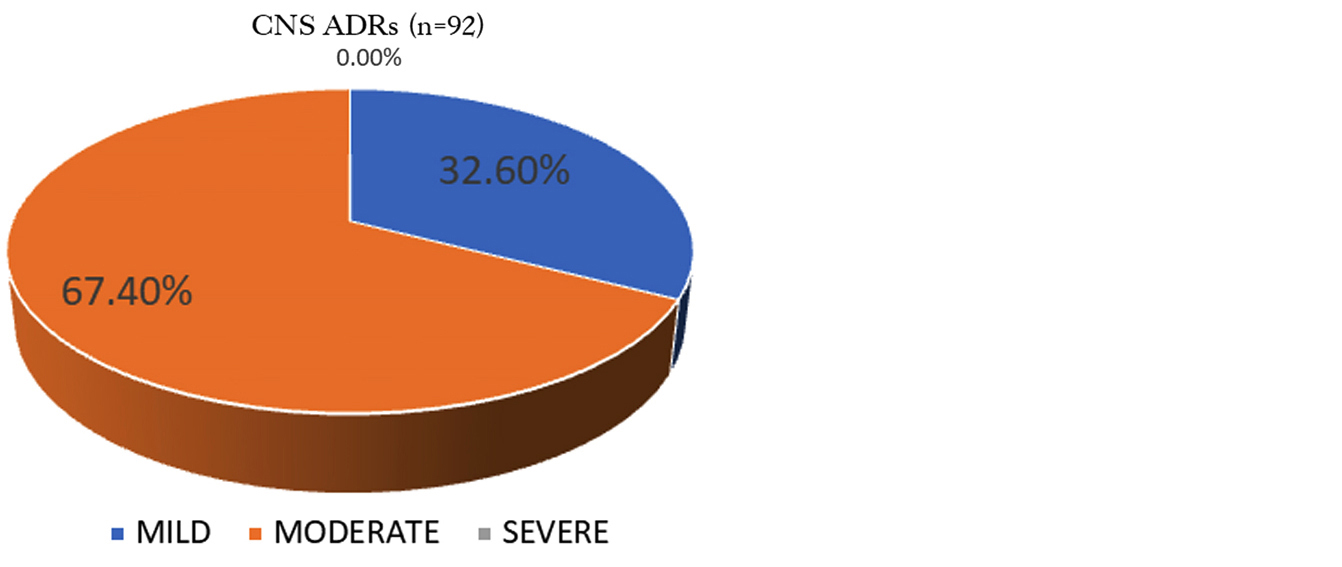
Figure 5: Severity assessment (Modified Hartwig scale).
Seriousness assessment of ADRs: All reported central nervous system related ADRs were assessed for seriousness as per criteria given by WHO. All CNS related ADRs were found non-serious in our study (Figure 6).
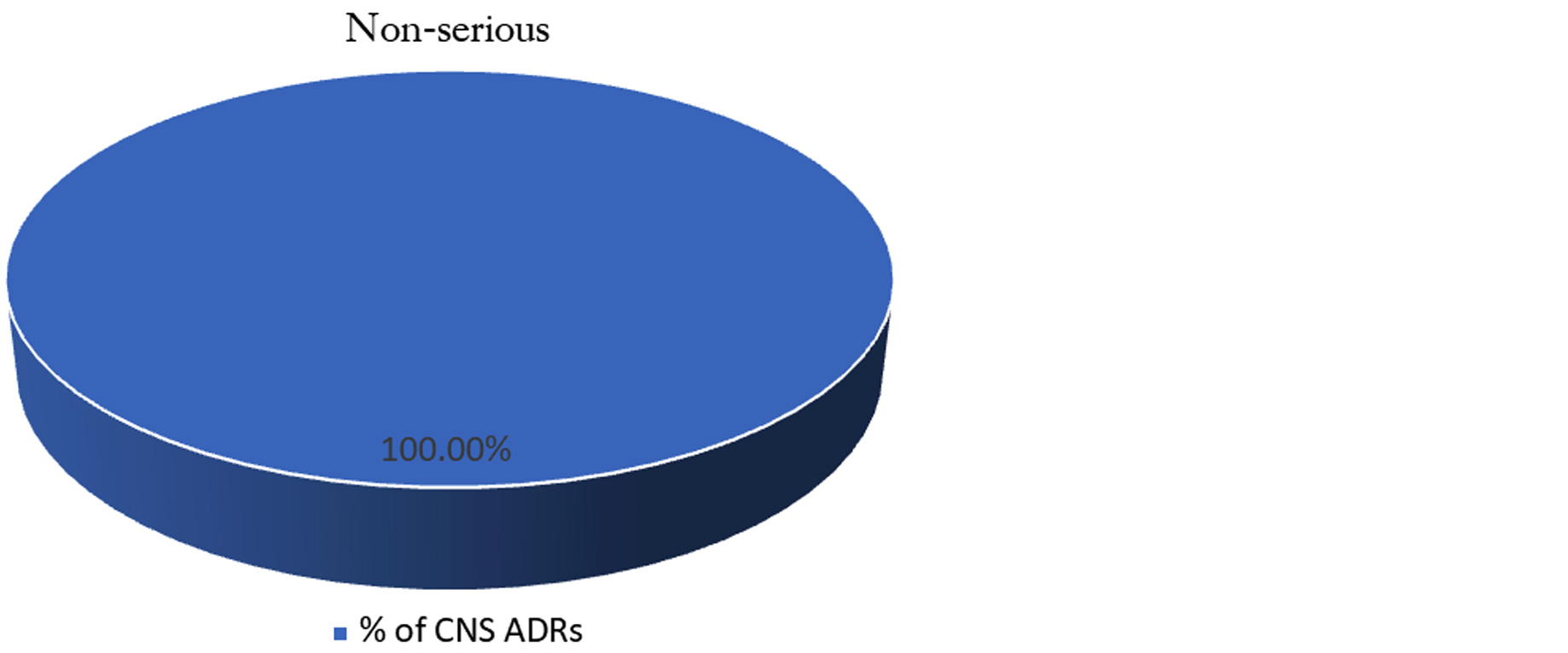
Figure 6: Seriousness assessment (W.H.O. Criteria).
Assessment of predictability of ADRs: All ADRs were analyzed for predictability according to modified guidelines given by the Council for International Organizations of Medical Sciences (CIOMS). Out of total 92 central nervous system ADRs all were predictable (Figure 7).
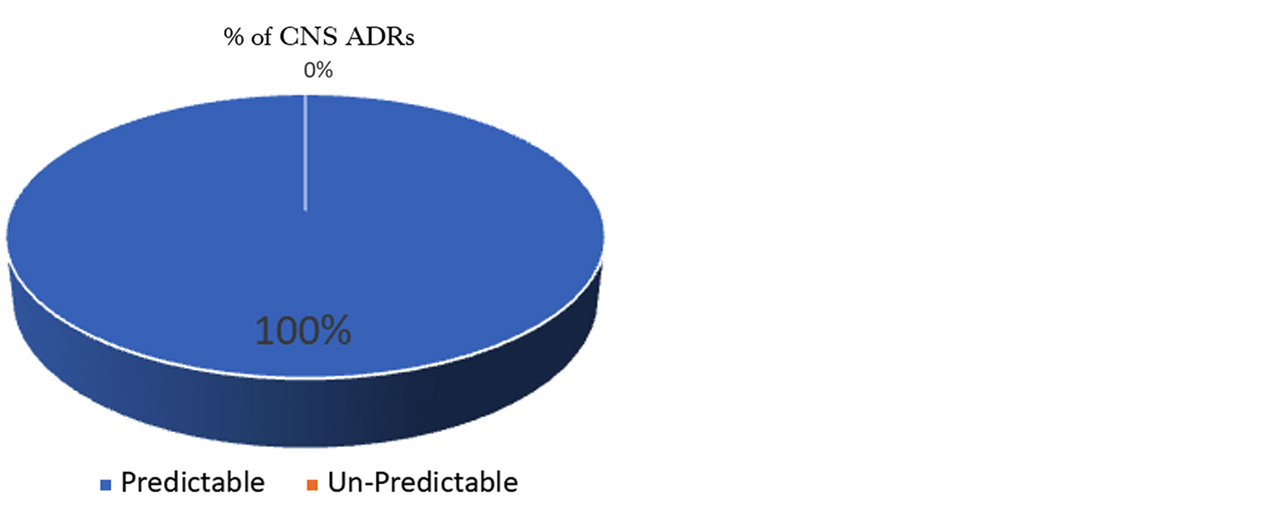
Figure 7: Predictability assessment (CIOMS Guidelines).
Assessment of outcome of ADRs: All ADRs were analyzed for outcome using WHO criteria. Out of 92 dermatological ADRs, the majority of cases were in recovered category followed by recovering and continuing category respectively (Figure 8).
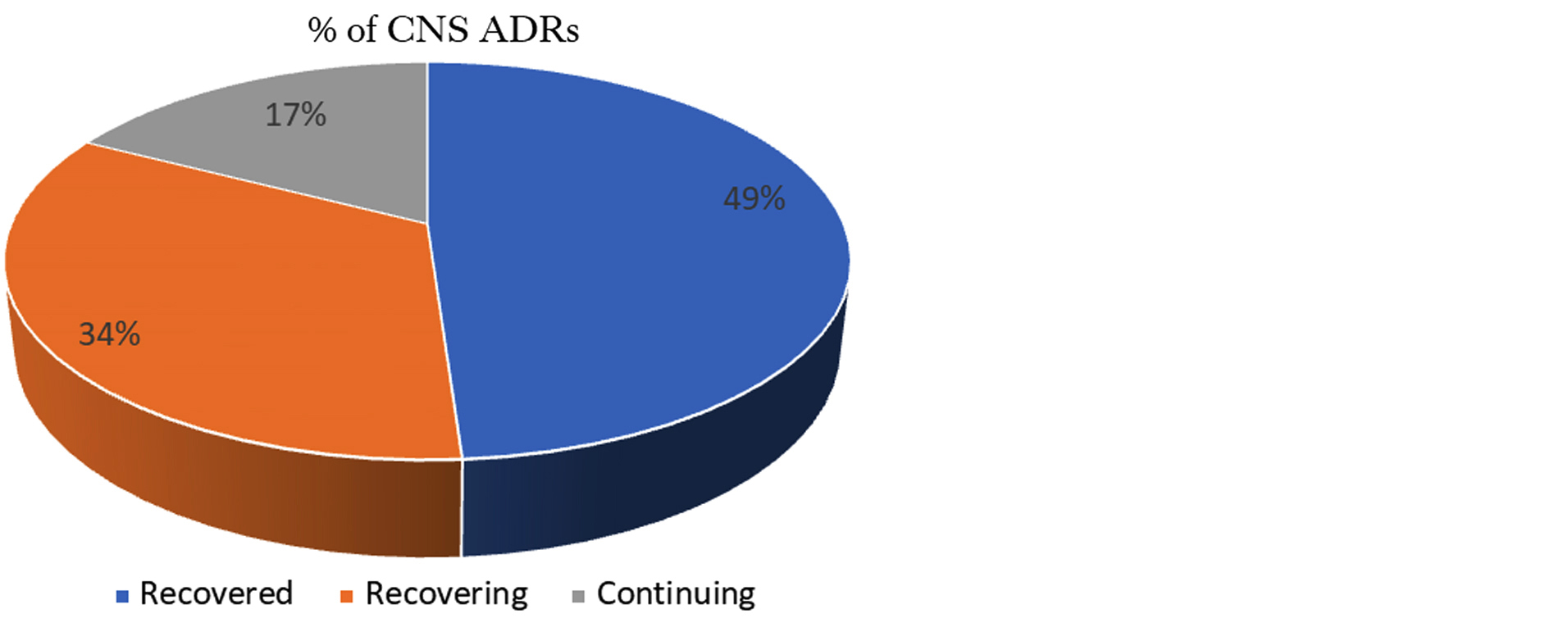
Figure 8: Outcome assessment (WHO criteria).
Assessment of preventability of ADRs: All ADRs were analyzed whether they are preventable or not. Modified Schumock Thornton Preventability Criteria was used for preventability assessment. All dermatological ADRs were Non preventable type in our study.
Discussion
In our study the mean age of patients who encountered CNS ADRs was more than the mean age who did not encounter CNS ADRs; which was found statistically significant. One plausible reason behind this finding may be that as the age increases the amount of water in the body decreases and amount of fat increases. So, the water-soluble drugs in the body get more concentrated and fat-soluble drugs remain for a long time in the body due to relatively more amount of fat in older age. Thus, both types of drugs cause more ADRs in older age.
In our study CNS toxicity was more common due to efavirenz containing regimen than the nevirapine containing and second line regimens; which was found statistically significant. This finding differs with study conducted by Wambura CA [15] in which no association was found between efavirenz containing regimen and CNS adverse effects. Most potential explanation behind finding in our study may be that efavirenz has active metabolite 8-hydroxy efavirenz which is neurotoxic while nevirapine has inactive metabolites. Other reasons are that efavirenz causes up-regulated production of pro-inflammatory cytokines (IL-1b and TNF-a) in blood cells exposed to pro-inflammatory stimuli, acts as a partial agonist of the serotonin receptors 5-HT2C & 5-HT2A, inhibit CK activity in different regions of the brain (cerebellum, hippocampus, striatum and cortex) and inhibition of mitochondrial complex IV (cytochrome c oxidase) in cerebral cortex, striatum and hippocampus, but not in cerebellum; whereas these effects are not seen or seen to a lesser degree with nevirapine.
Among central nervous system toxicity dizziness followed by headache were most common ADRs in our study which is in line with study done by Lihite et al [16] in which dizziness followed by insomnia were most common ADRs and differs from the study done by Manish Nagpal et al [17] in which insomnia followed by headache were most common ADRs.
In the current study patients with a CD4 count of below 250 are more likely to have CNS adverse events than those with a CD4 count of 250 or above. This may be explained by the fact that a CD4 count of below 250 have deteriorated medically and may not cope with the drug effect, or may have multiple comorbidities and opportunistic infections which they may consider and report as ADEs.
This study had several limitations. First, we did not assess the use of concomitant therapy beside ART, which may have caused drug reaction in patients. Second, some possible risk factors such as co-morbid conditions and baseline viral load number were not included in the present study. Last elucidation of underlying cellular and molecular mechanisms of central nervous system related ADRs due to anti-retroviral therapy is the need of future research.
Conclusion
Although the mortality from HIV has significantly decreased due to availability of highly active antiretroviral therapy, there has been a concurrent increase in the incidence of central nervous system related ADRs. Health-care providers should give attention to patients on efavirenz therapy to monitor for CNS adverse events, especially those patients who have low CD4 count, relatively overweight and older in age. We suggest that more stringent evaluation and monitoring should be carefully done in all HIV/AIDS patients who will receive and on efavirenz therapy, especially if they have the above predictors.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] UNAIDS Fact sheets AIDS. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
[2] PIB Press Release. Available at: https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1657057
[3] Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther. 2015; 12:6.
[4] Agu K, Ochei U, Oparah A, Onoh O. Treatment outcomes in patients receiving combination antiretroviral therapy in Central Hospital, Benin City, Nigeria. Trop J Pharm Res. 2010; 9:1–10.
[5] Agu KA, King RC, Isah MA, Iyaji PG. Medication adherence and risk factors for non-adherence among patients taking highly active antiretroviral therapy. West Afr J Pharm. 2011; 22:1.
[6] World Health Organization. Recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. Available at: https://iris.who.int/bitstream/handle/10665/273632/WHO-CDS-HIV-18.18 eng.pdf?ua=1
[7] Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001; 15:71–75.
[8] Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015; 70:2693–2708.
[9] Chelkeba L, Abdissa G. Assessment of ART adverse reactions and determinants at primary hospital in Ethiopia. Int J Basic Clin Pharmacol. 2013; 2:208–215.
[10] Scourfield A, Zheng J, Chinthapalli S, Waters L, Martin T, et al. Discontinuation of atripla as first-line therapy in HIV-1 infected individuals. AIDS. 2012; 26:1399–1401.
[11] National AIDS Control Organization. Antiretroviral Therapy Guidelines for HIV-infected Adults and Adolescents. New Delhi: Ministry of Health and Family Welfare, Government of India; May 2013. Available from: http://naco.gov.in/sites/default/files/Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents.pdf
[12] Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–245.
[13] Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992; 27:538.
[14] Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992; 49:2229–2232.
[15] Wambura CA. Assessment of the prevalence of central nervous system adverse reactions in adult patients taking efavirenz based regimens at Mbagathi District Hospital’s Comprehensive Care Centre. JKUAT. 2015.
[16] Reddy AK, Lihite RJ, Lahkar M, Choudhury U, Baruah SK. A study on adverse drug reactions in HIV infected patients at Art center of tertiary care hospital in Guwahati, India.Asian J Pharm Clin Res. 2013; 6:102–104.
[17] Nagpal M, Tayal V, Kumar S, Gupta U. Adverse drug reactions to antiretroviral therapy in HIV patients at a tertiary care hospital in India: A prospective observational study. Indian J Med Sci. 2010; 64:245–252.